Peritonitis
Peritonitis is inflammation of the peritoneum, typically caused by bacterial infection due to perforation of an abdominal viscus or contamination of the peritoneal cavity, presenting with severe abdominal pain, rigidity, and systemic sepsis.
Definition
Peritonitis refers to inflammation of the peritoneum — the serous membrane lining the abdominal cavity and covering the abdominal viscera [1][2][3]. The word itself breaks down from Greek: "peri" = around, "tonaion" = stretched (referring to the membrane), "-itis" = inflammation. So literally: inflammation of the membrane stretched around the abdominal contents.
It is one of the commonest surgical emergencies [1][2] and can rapidly become life-threatening. When the peritoneum is inflamed, it becomes oedematous, hyperaemic, and covered with fibrinous exudates [1][2]. This triggers a cascade of sequestration of large amounts of protein-rich fluid into the peritoneal cavity (third-spacing), leading to septicaemia, endotoxaemia, hypovolaemia and shock [1][2]. Left unchecked, this progresses to multi-organ failure and death.
Why is peritonitis so dangerous?
The peritoneum has an enormous surface area (~1.7 m², roughly equal to skin surface area). When inflamed, massive fluid shifts occur across this membrane into the peritoneal cavity ("third-space losses"). Simultaneously, bacteria and endotoxins are absorbed through the highly vascular peritoneal surface into the systemic circulation. This combination of hypovolaemia + sepsis is what kills patients.
Epidemiology and Risk Factors
Epidemiology
- Peritonitis is a leading cause of emergency surgical admissions worldwide.
- Spontaneous bacterial peritonitis (SBP) affects approximately 10–30% of hospitalized patients with cirrhotic ascites — highly relevant in Hong Kong given the prevalence of hepatitis B-related cirrhosis.
- CAPD-associated peritonitis is the leading cause of technique failure in peritoneal dialysis patients. Hong Kong has one of the highest rates of peritoneal dialysis utilization globally (>70% of dialysis patients are on PD), making this a particularly high-yield topic locally.
- Secondary peritonitis from perforated peptic ulcer (PPU), perforated appendicitis, and perforated diverticular disease constitutes the bulk of emergency surgical peritonitis cases.
- In the elderly, peritonitis is particularly dangerous because they are poor historians, may be confused or have dementia, history may be inaccurate (rely on care-provider), and peritoneal signs may be mild [1]. This demands a high index of suspicion in any elderly patient with abdominal pain, abdominal distension, fever, leucocytosis, acidosis, or sepsis of unexplained cause [1].
Risk Factors
The following are established risk factors for developing peritonitis [1][2]:
| Risk Factor | Mechanism |
|---|---|
| Ascites | Stagnant ascitic fluid provides an excellent culture medium; impaired peritoneal immune defences |
| Chronic liver disease / cirrhosis | Portal hypertension → ascites; impaired reticuloendothelial system (Kupffer cell dysfunction); decreased complement in ascitic fluid; bacterial translocation from gut |
| Malnutrition | Impaired immune function (reduced immunoglobulin, complement, and cell-mediated immunity) |
| Intra-abdominal malignancy | Tumour necrosis creates a nidus for infection; obstruction may lead to perforation; immunosuppression from cancer itself or chemotherapy |
| Chronic renal disease | Uraemic immunosuppression; PD catheter as a portal of entry |
| Immunosuppression | Steroids, chemotherapy, HIV — all reduce the ability to contain peritoneal infection |
| Splenectomy | Loss of splenic filtration function → susceptibility to encapsulated organisms (e.g., Streptococcus pneumoniae) |
Hong Kong Context
In HK, the particularly relevant causes of peritonitis include: (1) SBP in hepatitis B cirrhosis patients, (2) CAPD peritonitis given the high PD utilization, (3) perforated peptic ulcer (H. pylori + NSAID use in the aging population), and (4) perforated appendicitis / diverticulitis. TB peritonitis also remains relevant given Hong Kong's intermediate TB burden.
Anatomy and Function of the Peritoneum
Understanding peritoneal anatomy is essential to understanding patterns of fluid collection, infection spread, and surgical approach.
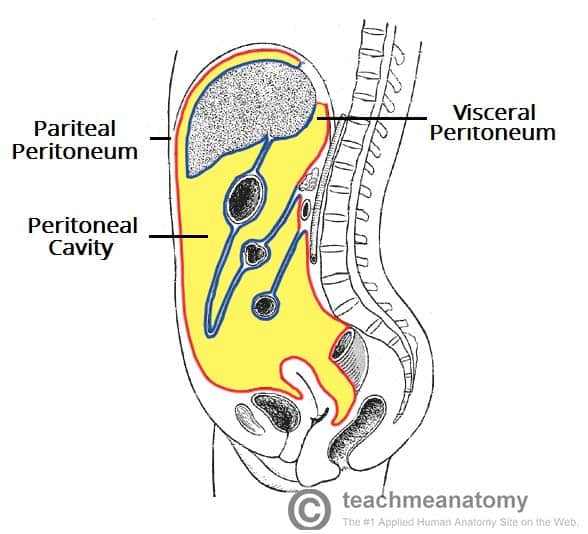
Structure
The peritoneum is a continuous serous membrane consisting of:
- Parietal peritoneum: Lines the inner surface of the abdominal and pelvic walls. Innervated by somatic nerves (intercostal nerves T7–T12, subcostal, iliohypogastric, ilioinguinal) → produces sharp, well-localised pain when inflamed.
- Visceral peritoneum: Covers the abdominal viscera. Innervated by autonomic (visceral) nerves → produces dull, poorly-localised, midline pain when inflamed.
This dual innervation explains a classic clinical pattern: early visceral peritoneal irritation (e.g., early appendicitis) causes vague periumbilical pain, but once the parietal peritoneum is involved, pain becomes sharp and localised to the right iliac fossa.
Key Peritoneal Spaces
Knowing peritoneal spaces helps you predict where fluid/pus collects:
- Morison's pouch (hepatorenal recess): The most dependent part of the peritoneal cavity in a supine patient. This is where you look for free fluid on FAST ultrasound [3].
- Right and left subphrenic spaces: Subdiaphragmatic abscesses collect here (e.g., post-splenectomy, perforated peptic ulcer).
- Right and left paracolic gutters: The right paracolic gutter communicates directly with the pelvis and the right subphrenic space — this is why a perforated appendix can cause a right subphrenic abscess (pus tracks up the right paracolic gutter).
- Left infra-mesocolic and right infra-mesocolic spaces: Divided by the root of the small bowel mesentery.
- Pelvis (pouch of Douglas / rectovesical pouch): The most dependent part when the patient is upright. Digital rectal examination can detect pelvic peritonitis as tenderness or a boggy mass here.
Retroperitoneal Organs (NOT covered by peritoneum — important for differential)
These structures, when inflamed, do not cause classical peritoneal signs initially [3]:
- Kidneys, adrenals, ureters
- Aorta / IVC
- Duodenum (D2, D3), ascending and descending colon
- Pancreas (except the tail)
Clinical Pearl
A ruptured AAA or acute pancreatitis can mimic peritonitis but may lack classical peritoneal signs initially because these are retroperitoneal structures. However, if pancreatic enzymes or blood leaks into the peritoneal cavity, secondary peritoneal irritation occurs.
Function of the Peritoneum
- Friction reduction: Mesothelial cells secrete a thin layer of serous fluid (~50–100 mL) that lubricates visceral surfaces.
- Immune defence: Contains macrophages, lymphocytes, and mast cells; the omentum ("policeman of the abdomen") migrates to sites of inflammation to wall off infection.
- Fluid and solute exchange: The large surface area (~1.7 m²) allows significant absorption — this is why peritoneal dialysis works, but also why toxins/bacteria are rapidly absorbed systemically.
- Support and compartmentalization: Mesenteries and ligaments suspend organs and create compartments that can temporarily localise infection.
Etiology
Classification-Based Approach to Etiology
Peritonitis is classified in different ways [1][2]:
- Localized vs. generalized (diffuse) — based on extent
- Bacterial vs. chemical — based on etiology
- Primary vs. secondary vs. tertiary — based on source
This last classification is the most clinically useful and exam-relevant:
1. Primary Peritonitis
Definition: Ascitic fluid infection without a surgically treatable intra-abdominal source of infection [1][2][3]
The infection reaches the peritoneum via haematogenous spread, lymphatic spread, or transmural migration (bacterial translocation) from the gut — there is NO perforation or breach in the GI tract.
a) Spontaneous Bacterial Peritonitis (SBP)
- Who gets it: Patients with cirrhosis and ascites (especially decompensated liver disease) [2][3]
- Pathophysiology:
- Portal hypertension → intestinal mucosal oedema → impaired mucosal barrier → bacterial translocation (bacteria cross from gut lumen into mesenteric lymph nodes and portal blood)
- Cirrhosis → decreased hepatic reticuloendothelial function (Kupffer cells cannot clear bacteria) → bacteraemia
- Low complement and low opsonic activity in ascitic fluid (ascitic fluid protein < 1 g/dL has poor opsonic capacity) → bacteria proliferate unchecked in ascites
- Organisms: Strep. pneumoniae, Group A Streptococcus, Enteric organisms (especially E. coli, Klebsiella pneumoniae) [1][2]
- Typically Gram-negative enteric organisms predominate, followed by Gram-positive cocci
b) Tuberculous Peritonitis
- Considered rare in developed countries but relevant in Hong Kong (intermediate TB burden) [1]
- Presentation may be non-specific: low-grade fever, weight loss [1]
- Insidious onset of abdominal pain; peritoneal signs not florid [1]
- Peritoneal fluid: AFB smear often negative; culture takes 4–6 weeks (could be falsely negative) [1]
- Diagnosis is often made by laparoscopy and biopsy of peritoneum — showing caseating granulomata [1]
- Pathophysiology: Haematogenous spread from pulmonary or extrapulmonary TB; can also result from direct spread from infected mesenteric lymph nodes or fallopian tubes
- The ascitic fluid typically has high protein ( > 2.5 g/dL), high lymphocyte count (lymphocyte-predominant), and elevated adenosine deaminase (ADA > 39 U/L)
c) CAPD-Associated Peritonitis
- Primary peritonitis presenting with fever, abdominal pain and turbid PD fluid [2]
- Organisms [1][2]:
- Gram +ve: Staphylococcus sp. (particularly coagulase-negative Staphylococci — from skin flora around catheter exit site), S. aureus
- Gram -ve: E. coli, Campylobacter, Pseudomonas aeruginosa
- Mycobacterium tuberculosis
- Fungal species
- Risk factors for CAPD peritonitis [2]:
- Catheter-associated infection (exit-site and tunnel infections)
- Lack of sanitary conditions / unhygienic home environment
- Poor dexterity (e.g., diabetic retinopathy → poor hand-eye coordination during exchanges)
- Underlying GI pathology
- Recent invasive procedures (colonoscopy, cystoscopy)
- Nasal carriage of S. aureus
- Constipation (increased bacterial translocation)
- Smoking
Exam Pearl
SBP is MONOmicrobial. If you culture MULTIPLE organisms from the ascitic fluid of a cirrhotic patient, think secondary peritonitis (bowel perforation) rather than SBP — this changes management completely (surgery vs. antibiotics alone).
2. Secondary Peritonitis
Definition: Ascitic fluid infection with a surgically treatable intra-abdominal source of infection [1][2][3]
This accounts for most peritonitis cases [1]. The peritoneal cavity is contaminated by GI contents, bile, urine, or pancreatic juice through a breach in a hollow viscus or direct spread from an infected organ.
Could be localised (e.g., intra-abdominal abscess) or diffuse [1]
Could be preceded by chemical peritonitis (e.g., gastric juice, bile, pancreatic juice, urine & blood) — chemical irritation then becomes secondarily infected [1][2]
Causes (by mechanism)
| Mechanism | Examples |
|---|---|
| Severe inflammation of abdominal organ | Diverticulitis, cholecystitis, appendicitis [1] |
| Perforation of GI tract (spontaneous, trauma, iatrogenic) | PPU, perforated appendix, perforated diverticular disease, perforated colon cancer, Boerhaave syndrome, traumatic bowel injury, colonoscopic perforation [1] |
| Anastomotic leakage | Post-operative leak from bowel anastomosis [1] |
| Ischaemia of abdominal organ | Mesenteric ischaemia → bowel gangrene → perforation [1] |
| Incarcerated / strangulated hernia | Bowel within hernia becomes ischaemic → gangrene → perforation |
Microbiology
Polymicrobial (mixed aerobic + anaerobic flora reflecting GI tract contents) [1][2]:
- Gram-negative: E. coli, Enterobacter, Proteus, Pseudomonas [1]
- Gram-positive: Streptococci, Enterococci [1]
- Anaerobes: Bacteroides [1]
The microbiological profile depends on the level of GI tract perforation:
- Stomach/duodenum: Low bacterial load (acid environment); chemical peritonitis predominates initially. Organisms: Streptococci, Lactobacilli, Candida
- Small bowel: Intermediate bacterial load. Organisms: Gram-negatives + some anaerobes
- Perforation of small bowel is specifically highlighted as having the polymicrobial profile listed above [1]
- Colon: Highest bacterial load (10¹¹ organisms/mL). Heavily polymicrobial including abundant anaerobes (Bacteroides fragilis)
Chemical → Bacterial Peritonitis Timeline
When gastric juice (pH ~1-2) leaks into the peritoneum from a PPU, the initial insult is CHEMICAL — causing intense inflammation but initially sterile. Within 6-12 hours, bacteria colonise the peritoneal fluid and it becomes secondary bacterial peritonitis. This is why early surgery for PPU (within 6 hours) has better outcomes.
3. Tertiary Peritonitis
Definition: Persistent peritonitis after adequate initial therapy [3] — i.e., peritonitis that persists or recurs > 48 hours after apparently adequate surgical source control and antibiotic therapy.
- Caused by opportunistic infections with normally non-pathogenic gut flora [2][3]:
- Staphylococcus infection
- Enterococcus infection
- Candida infection
- Other: coagulase-negative Staphylococci, Enterobacter, Pseudomonas
- Associated with prolonged use of antibiotics in persistent intra-abdominal infection [2] → selects for resistant/opportunistic organisms
- Typically occurs in ICU patients, immunocompromised hosts, or those with ongoing source of contamination
- Carries the worst prognosis of all three types (mortality 30–60%)
Pathophysiology
Understanding the pathophysiology of peritonitis is crucial because it explains every clinical feature and guides treatment:
Sequence of Events
Detailed Pathophysiology
-
Initial insult: Bacteria, chemical irritants (gastric acid, bile, pancreatic enzymes), or both contact the peritoneal mesothelium.
-
Inflammatory response: Mesothelial cells release cytokines (IL-1, IL-6, TNF-α) and chemokines → recruitment of neutrophils and macrophages → peritoneal hyperaemia and oedema.
-
Fibrinous exudate formation: Increased vascular permeability leads to exudation of protein-rich fluid containing fibrinogen. Fibrinogen is converted to fibrin, creating fibrinous adhesions. These serve a dual purpose:
- Beneficial: Wall off infection (localised peritonitis / abscess formation — the omentum contributes as the "abdominal policeman")
- Detrimental: Can cause bowel obstruction (adhesions), trap bacteria in pockets inaccessible to antibiotics
-
Third-space fluid losses: The inflamed peritoneal surface (1.7 m²) leaks massive amounts of protein-rich fluid into the peritoneal cavity → intravascular volume depletion → hypovolaemia → tachycardia → hypotension → shock.
-
Paralytic ileus: Peritoneal inflammation → reflex inhibition of intestinal motility via:
- Sympathetic overactivation (splanchnic nerves)
- Local inflammatory mediators (prostaglandins, nitric oxide)
- Direct irritation of bowel serosa → Bowel distension → further fluid sequestration within the bowel lumen (yet more third-spacing)
-
Systemic absorption: The peritoneum's vast surface area rapidly absorbs bacteria and endotoxins → bacteraemia / endotoxaemia → systemic inflammatory response syndrome (SIRS) → sepsis → septic shock → multi-organ dysfunction.
-
Metabolic consequences:
- Massive protein loss (albumin leaks into peritoneal cavity) → hypoalbuminaemia
- Metabolic acidosis (tissue hypoperfusion + lactate production)
- Respiratory compromise (diaphragmatic splinting from pain + abdominal distension pushing diaphragm up → basal atelectasis)
Classification
Summary Table of Classification Systems
| Classification Axis | Types | Key Feature |
|---|---|---|
| By source | Primary / Secondary / Tertiary | Whether there is a surgically treatable source [1][2][3] |
| By extent | Localised vs. Generalized (Diffuse) | Localised = contained (e.g., abscess); Generalized = widespread peritoneal contamination [1][2] |
| By etiology | Bacterial vs. Chemical | Chemical (e.g., gastric acid, bile, pancreatic juice) often precedes bacterial infection [1][2] |
Hinchey Classification (for Diverticulitis-Related Peritonitis)
This is specifically geared towards choosing the surgical approach for complicated diverticulitis [4]:
| Stage | Description | Relevance to Peritonitis |
|---|---|---|
| I | Pericolic / mesenteric abscess | Localised, contained |
| II | Walled-off pelvic abscess | Localised, but larger |
| III | Generalised purulent peritonitis | Free pus in peritoneal cavity |
| IV | Generalised faecal peritonitis | Free faecal contamination — worst prognosis |
Clinical Features
The clinical features of peritonitis are a direct reflection of the underlying pathophysiology. I'll separate them into symptoms (what the patient tells you) and signs (what you find on examination), with the pathophysiological basis explained inline.
Symptoms
| Symptom | Pathophysiological Basis |
|---|---|
| Abdominal pain — the hallmark of peritonitis [2] | Inflammation of the parietal peritoneum (somatic innervation → sharp, well-localised pain). Diffuse, continuous, and burning in nature [2]. Initially localised (to the primary pathology, e.g., RIF in appendicitis) and later spreading as peritonitis generalises [1]. Exacerbated by movement and coughing because any movement stretches or jarrs the inflamed parietal peritoneum [1][2]. Patients characteristically lie still (unlike colicky pain where patients writhe around). |
| Fever | Fever is the most common clinical manifestation [2]. Cytokines (IL-1, IL-6, TNF-α) released from the inflamed peritoneum act on the hypothalamic thermoregulatory centre → ↑ temperature set-point. |
| Hypothermia (in advanced disease) | Mildly hypothermic in patients with advanced disease [2]. Indicates decompensated sepsis — the body can no longer mount a febrile response (immune exhaustion, cardiovascular collapse). This is an ominous sign. |
| Altered mental status | Development of delirium, confusion and cognitive slowing [2]. Caused by infection (septic encephalopathy — cytokines cross the blood-brain barrier, alter neurotransmission) and hepatic decompensation (in cirrhotic patients — ammonia accumulation) [2]. |
| Nausea and vomiting | Peritoneal irritation → vagal afferent stimulation → vomiting centre in medulla. Also due to paralytic ileus (retrograde accumulation of GI contents). |
| Anorexia | Systemic inflammatory cytokines suppress appetite centres in the hypothalamus. |
| Diarrhoea | Alteration in gut flora with overgrowth of one organism, usually E. coli [2]. Also, pelvic peritonitis can irritate the rectum directly, causing tenesmus and frequent small-volume stools. |
| Inability to pass flatus | Paralytic ileus → functional obstruction → no passage of gas. |
| Abdominal distension | Patient notices their abdomen becoming progressively swollen — due to ileus (gas and fluid accumulation in bowel) and peritoneal fluid accumulation. |
Signs
General / Systemic Signs
| Sign | Pathophysiological Basis |
|---|---|
| Fever / Hypothermia | As above — cytokine-mediated vs. septic decompensation [1] |
| Tachycardia | Compensatory response to hypovolaemia (third-space losses) and pain. Also a feature of SIRS/sepsis (catecholamine surge). [1] |
| Tachypnoea | (1) Metabolic acidosis → respiratory compensation (Kussmaul breathing); (2) Abdominal distension pushing diaphragm up → reduced tidal volume → compensatory ↑ RR; (3) Pain-related splinting of respiration; (4) SIRS criterion. [1] |
| Hypotension | Hypovolaemia from third-space fluid losses + sepsis-induced vasodilation (nitric oxide-mediated) + myocardial depression from septic cardiomyopathy. [2] |
| Septic shock | The end-stage haemodynamic consequence: distributive shock (vasodilation) superimposed on hypovolaemic shock. [1] |
| Altered mental status / confusion | Reduced cerebral perfusion (shock) + septic encephalopathy + hepatic encephalopathy in cirrhotics. [1][2] |
| Dehydration signs | Dry mucous membranes, sunken eyes, reduced skin turgor, oliguria — all reflecting third-space losses. |
Abdominal Signs
| Sign | Pathophysiological Basis |
|---|---|
| Tenderness | Inflammation of parietal peritoneum → somatic nerve stimulation → pain on palpation. Initially localised to the site of primary pathology; becomes diffuse as peritonitis generalises. [1] |
| Rebound tenderness (Blumberg's sign) | When you release pressure after deep palpation, the inflamed peritoneum springs back and is stimulated → sharp pain. This is a peritoneal sign indicating parietal peritoneal irritation. [1] |
| Guarding | Involuntary contraction of the abdominal wall muscles overlying the inflamed peritoneum — a protective reflex mediated by the spinal reflex arc (peritoneal irritation → afferent signal via intercostal nerves → spinal cord → efferent motor response → muscle contraction). [1] |
| Board-like rigidity | The extreme form of guarding seen in diffuse peritonitis — the entire abdominal wall is rigid and "hard as a board" due to generalised involuntary muscle spasm. Classic for a perforated viscus with generalised peritonitis. [2] |
| Abdominal distension | Paralytic ileus → gas and fluid accumulate in dilated bowel loops. Also, free fluid/pus in the peritoneal cavity. [2] |
| Shifting dullness | Large volume of free peritoneal fluid (ascites/pus/blood) shifts with gravity when the patient changes position → dullness shifts. [2] |
| Absence of bowel sounds (paralytic ileus) | Peritoneal inflammation causes reflex inhibition of bowel motility — sympathetic overactivation inhibits peristalsis; local inflammatory mediators (prostaglandins, NO) paralyse smooth muscle. A "silent abdomen" on auscultation is ominous. [1][2] |
| Percussion tenderness | Tapping the abdomen jars the inflamed peritoneum → pain. A gentler way to elicit peritoneal irritation than deep palpation. |
| Digital rectal examination — tenderness | Pelvic peritonitis causes tenderness on palpation of the pouch of Douglas / rectovesical pouch via DRE. May feel a boggy, tender mass if a pelvic abscess is present. |
The Classic Peritoneal Triad: T + G + R
Special Considerations
Peritonitis in the elderly deserves special mention [1]:
- Poor historian — may be confused or have dementia
- History may be inaccurate → rely on care-provider
- Peritoneal signs may be mild — elderly patients have thinner abdominal musculature (less guarding), reduced inflammatory response, and may be on analgesics/steroids that mask signs
- Need a high index of suspicion: any elderly patient with abdominal pain, abdominal distension, fever, leucocytosis, acidosis, or sepsis of unexplained cause should be investigated for peritonitis [1]
Exam Trap: Absence of Peritoneal Signs ≠ Absence of Peritonitis
Several groups may NOT show classical peritoneal signs despite having peritonitis: (1) Elderly/demented patients, (2) Immunosuppressed patients (steroids suppress inflammation), (3) Patients with ascites (fluid cushion prevents direct peritoneal contact), (4) Patients on strong analgesics. Always correlate with blood tests (leucocytosis, raised CRP/lactate) and imaging.
Symptoms and Signs of the Primary Pathology
Peritonitis clinical features include symptoms and signs of the primary pathology [1]. This means you will also see features specific to whatever caused the peritonitis:
- PPU: Sudden-onset epigastric pain, preceding dyspepsia, NSAID/steroid use
- Appendicitis: Migratory RIF pain, anorexia, Rovsing's/psoas/obturator signs
- Diverticulitis: LIF pain (or RIF in Asian right-sided diverticular disease), altered bowel habit
- Cholecystitis: RUQ pain, Murphy's sign, jaundice
- Pancreatitis: Epigastric pain radiating to back, raised amylase/lipase
- SBP: History of liver disease, jaundice, ascites, encephalopathy
- CAPD peritonitis: Turbid PD fluid, catheter exit-site erythema/discharge
Peritoneal Fluid Analysis
Peritoneal fluid analysis is critical for distinguishing the type and cause of peritonitis [1][2]:
| Parameter | What It Tells You |
|---|---|
| Character: serous, blood-stained, purulent, bile-stained, faeculent | Serous = SBP or early; Blood-stained = trauma/malignancy/pancreatitis; Purulent = established bacterial peritonitis; Bile-stained = perforated GB or biliary injury; Faeculent = perforated bowel [1] |
| Cell counts: Neutrophil count > 500/μL | Indicative of bacterial peritonitis. SBP diagnosed at PMN ≥ 250 cells/mm³ [1][2] |
| Low glucose, high protein, high LDH compared to serum | Suggests secondary peritonitis (bacteria consume glucose; damaged cells release LDH and protein). Runyon's criteria. [1] |
| Gram stain | Rapid identification of organism morphology. Positive in ~25% of SBP (low sensitivity). |
| Cultures: aerobic, anaerobic, AFB, fungal | Definitive identification. Inoculate ascitic fluid into blood culture bottles at bedside to improve yield. [1] |
| Amylase | ↑ Amylase in peritoneal fluid → perforated gut (duodenal/small bowel) or pancreatitis [1][3] |
| Creatinine | ↑ Creatinine in peritoneal fluid (higher than serum) → urinary tract injury / bladder perforation [1] |
↑ Amylase / bile-stained / faeculent peritoneal fluid indicates perforated GI tract [3]
Summary: Connecting Pathophysiology to Clinical Features
High Yield Summary
Definition: Peritonitis = inflammation of the peritoneum; a surgical emergency.
Classification:
- Primary: No surgical source (SBP, CAPD, TB peritonitis) — monomicrobial
- Secondary: Surgical source present (perforation, ischaemia, inflammation) — polymicrobial — accounts for most cases
- Tertiary: Persistent despite adequate therapy — opportunistic organisms (Candida, Enterococcus, Staph)
Also classified by: Localised vs. diffuse; Bacterial vs. chemical
Risk factors: Ascites, CLD, malnutrition, malignancy, CKD, immunosuppression, splenectomy
Pathophysiology cascade: Peritoneal insult → hyperaemia + oedema + fibrinous exudates → third-space loss (hypovolaemia) + bacterial absorption (septicaemia) → shock + MOF
Key clinical features:
- Symptoms: Burning abdominal pain (worse with movement/cough), fever (hypothermia in advanced disease), altered mental status, nausea/vomiting, diarrhoea
- Signs: Tenderness + Guarding + Rebound (T+G+R) = peritoneal signs; board-like rigidity; absent bowel sounds; tachycardia, hypotension, tachypnoea
- Elderly: Mild peritoneal signs — high index of suspicion needed
Peritoneal fluid analysis: Character, cell count (PMN > 500), glucose/protein/LDH, Gram stain, cultures (aerobic/anaerobic/AFB/fungal), amylase, creatinine
If free gas on erect CXR + florid peritoneal signs → exploratory laparotomy
Active Recall - Peritonitis (Definition, Epidemiology, Anatomy, Etiology, Pathophysiology, Classification, Clinical Features)
1. What are the three classifications of peritonitis by source, and what distinguishes each?
Show mark scheme
Primary = no surgically treatable intra-abdominal source (e.g. SBP, CAPD, TB); Secondary = surgically treatable source present (e.g. perforation, ischaemia, inflammation) - accounts for most cases; Tertiary = persistent peritonitis after adequate initial therapy - opportunistic organisms (Candida, Enterococcus, Staph).
2. Why does peritonitis cause hypovolaemia and shock? Explain the pathophysiological cascade.
Show mark scheme
Inflamed peritoneum (surface area ~1.7 m2) becomes hyperaemic, oedematous, covered with fibrinous exudates. Massive protein-rich fluid sequestered into peritoneal cavity (third-spacing). Intravascular volume depleted. Also paralytic ileus causes further fluid sequestration in dilated bowel. Combined with sepsis-induced vasodilation leads to hypovolaemic + distributive shock.
3. What is the classic peritoneal triad on examination, and what is the pathophysiological basis of guarding?
Show mark scheme
Tenderness + Guarding + Rebound (T+G+R). Guarding = involuntary contraction of abdominal wall muscles via spinal reflex arc: inflamed parietal peritoneum stimulates somatic afferents (intercostal nerves) to spinal cord, which sends efferent motor signals causing overlying muscle contraction as a protective reflex.
4. SBP is monomicrobial while secondary peritonitis is polymicrobial. Name the common organisms for each and explain why this distinction matters clinically.
Show mark scheme
SBP: monomicrobial - E. coli, Klebsiella, Streptococcus pneumoniae, Group A Strep. Secondary: polymicrobial - Gram-neg (E. coli, Enterobacter, Proteus, Pseudomonas), Gram-pos (Streptococci, Enterococci), Anaerobes (Bacteroides). If multiple organisms cultured from ascitic fluid in a cirrhotic patient, suspect secondary peritonitis (bowel perforation) rather than SBP - changes management from antibiotics alone to surgery.
5. Why are elderly patients with peritonitis particularly dangerous? List the clinical pitfalls.
Show mark scheme
Poor historians, may be confused or demented. History may be inaccurate, need to rely on care-providers. Peritoneal signs may be mild (thinner abdominal muscles, reduced inflammatory response, may be on steroids/analgesics). Need high index of suspicion: look for abdominal pain, distension, fever, leucocytosis, acidosis, unexplained sepsis. Delayed diagnosis leads to higher morbidity and mortality.
6. In peritoneal fluid analysis, what does bile-stained fluid, raised amylase, and raised creatinine each indicate?
Show mark scheme
Bile-stained = biliary perforation or gallbladder injury. Raised amylase = perforated duodenum/small bowel or pancreatitis. Raised creatinine (higher than serum) = bladder perforation or urinary tract injury.
References
[1] Lecture slides: GC 195. Lower and diffuse abdominal pain RLQ problems; pelvic inflammatory disease; peritonitis and abdominal emergencies.pdf (p34–43) [2] Senior notes: felixlai.md (Peritonitis section, p738–743; CAPD peritonitis section, p866) [3] Senior notes: maxim.md (Section 2.5 Peritonitis) [4] Senior notes: felixlai.md (Hinchey classification, diverticular disease section, p637)
Differential Diagnosis of Peritonitis
Why Is the Differential Diagnosis of Peritonitis Important?
Peritonitis is not a single disease — it is a clinical syndrome (inflammation of the peritoneum) that can be caused by dozens of different conditions. When a patient presents with peritoneal signs (tenderness, guarding, rebound) [1], your job is twofold:
- Confirm peritonitis — could the peritoneal signs be mimicked by something else (e.g., abdominal wall pathology, retroperitoneal disease, medical causes)?
- Identify the underlying cause — because management differs dramatically (e.g., SBP = antibiotics alone vs. perforated viscus = emergency surgery).
The DDx is best approached systematically by thinking about what can irritate the peritoneum from first principles: (a) things that perforate/leak into the peritoneal cavity, (b) organs that inflame and involve the adjacent peritoneum, (c) primary peritoneal disease, and (d) "mimics" — conditions that look like peritonitis but aren't.
Framework for Differential Diagnosis
I'll organize this in two layers:
Layer 1 — Differentiating BETWEEN the causes of peritonitis (i.e., once you know the peritoneum is inflamed, what's the aetiology?)
Layer 2 — Differentiating peritonitis FROM its mimics (i.e., conditions that present similarly but the peritoneum is not the primary problem)
Layer 1: Causes of Peritonitis — Differential by Type
This is essentially the aetiological differential: "What caused the peritonitis?"
A. Primary Peritonitis
| Condition | Key Differentiating Features |
|---|---|
| Spontaneous bacterial peritonitis (SBP) | History of liver cirrhosis and ascites [1][2]. Fever, abdominal pain, altered mental status. Ascitic fluid PMN ≥ 250 cells/mm³, monomicrobial culture. No free gas on imaging. Risk factors: ascites, malnutrition, immunosuppression, CLD, CKD, splenectomy, intra-abdominal malignancy [1]. |
| Tuberculous peritonitis | Presentation may be non-specific: low-grade fever, weight loss, insidious onset of abdominal pain [1]. Peritoneal signs not florid [1]. Ascitic fluid is lymphocyte-predominant (not neutrophil-predominant like SBP), high protein ( > 2.5 g/dL), elevated ADA. AFB smear often negative; culture takes 4–6 weeks (could be falsely negative) [1]. Diagnosis often made by laparoscopy and biopsy of peritoneum [1]. HK context: intermediate TB burden, consider in immigrants/immunosuppressed. |
| CAPD-associated peritonitis | Patient on peritoneal dialysis with turbid PD effluent, abdominal pain ± fever [2]. PD fluid WBC ≥ 100/mm³ with > 50% PMN. Look for exit-site infection (erythema, discharge at catheter site). Organisms: coagulase-negative Staph (from skin), S. aureus, Gram-negatives [2]. |
SBP vs. Secondary Peritonitis in a Cirrhotic Patient — Critical Distinction
Both can present identically in a cirrhotic patient with ascites. If you culture multiple organisms from the ascitic fluid, or if Runyon's criteria are met (protein > 1 g/dL, glucose < 50 mg/dL, LDH > ULN for serum [2]), think secondary peritonitis — this patient needs surgery, not just antibiotics. Missing a perforation in a cirrhotic is a classic fatal error.
B. Secondary Peritonitis — The Main Differentials
Accounts for most peritonitis [1]. The differential here is essentially: which organ has perforated, become ischaemic, or is severely inflamed?
By Mechanism: Perforation
| Condition | Key Differentiating Features |
|---|---|
| Perforated peptic ulcer (PPU) | Sudden-onset severe epigastric pain → rapidly becomes diffuse. History of dyspepsia, NSAID/steroid use, H. pylori. Board-like rigidity (chemical peritonitis from gastric acid). Free gas under diaphragm on erect CXR is the classic finding. Chemical peritonitis preceded by bacterial peritonitis [1] — gastric acid initially sterile, secondary infection follows in 6–12 hours. |
| Perforated appendicitis | Initial periumbilical pain → migrating to RIF (the classic visceral → somatic shift). Fever, anorexia, nausea. McBurney's point tenderness, Rovsing's sign, psoas sign [5]. If perforation occurs → generalised peritonitis with diffuse tenderness and guarding. Peak age 20s–30s [2]. Grade 5: Perforated with diffuse peritonitis [2]. |
| Perforated diverticulitis | LIF pain (or RIF in Asian population with right-sided diverticular disease [2]). History of altered bowel habit, older age (mean 63 years [2]). CT: bowel wall thickening, pericolonic fat stranding ± free gas/fluid. Hinchey III = generalised purulent peritonitis; Hinchey IV = generalised faecal peritonitis [2][6]. |
| Perforated colorectal cancer | May present acutely with peritonitis. History of weight loss, altered bowel habit, PR bleeding, iron-deficiency anaemia. CT: mass lesion with perforation. Similar features to diverticulitis on imaging — CRC can only be excluded with colonoscopy after resolution of acute inflammation [2]. |
| Perforations of GI tract: spontaneous, trauma, iatrogenic | Post-colonoscopy perforation (iatrogenic), blunt/penetrating abdominal trauma, spontaneous perforation of stercoral ulcer in severe constipation [1]. |
| Boerhaave syndrome | Perforation of oesophagus (usually distal) after forceful vomiting. Severe chest/epigastric pain, subcutaneous emphysema, Hamman's sign (mediastinal crunch). Leads to mediastinitis ± peritonitis if perforation is below diaphragm. |
By Mechanism: Severe Inflammation
| Condition | Key Differentiating Features |
|---|---|
| Cholecystitis | RUQ pain, fever, Murphy's sign (+ve). Inflammatory process may remain localised or spread to involve the peritoneum (localised peritonitis → diffuse if perforated). USG: thickened GB wall, pericholecystic fluid, gallstones [1]. |
| Cholangitis | Charcot's triad: fever + jaundice + RUQ pain. Reynold's pentad adds hypotension + confusion (sepsis). Biliary obstruction → ascending infection. |
| Pancreatitis | Epigastric pain radiating to back, relieved by leaning forward. Markedly elevated serum amylase/lipase ( > 3× ULN). Could be preceded by chemical peritonitis [1] — pancreatic enzymes leak into the peritoneal cavity causing chemical irritation. Grey-Turner (flank ecchymosis) and Cullen (periumbilical ecchymosis) signs in severe haemorrhagic pancreatitis [5]. |
| Diverticulitis (uncomplicated) | LIF pain/tenderness, low-grade fever, altered bowel habit. Severe inflammation of abdominal organ causing localised peritoneal irritation without free perforation [1]. |
| Appendicitis (uncomplicated) | RIF pain, low-grade fever, anorexia. Localised peritonitis in RIF before perforation occurs [1]. |
By Mechanism: Ischaemia
| Condition | Key Differentiating Features |
|---|---|
| Ischaemic bowel (acute mesenteric ischaemia) | "Pain out of proportion to examination" in the early stages (visceral pain with minimal signs). Later → bowel infarction → full peritonitis with guarding, rigidity. Risk factors: AF (embolic), atherosclerosis, low-flow states. Markedly elevated lactate and metabolic acidosis. Ischaemia of abdominal organ e.g. bowel [1]. |
| Strangulated / incarcerated hernia | Irreducible, tender lump at hernia site (inguinal, femoral, incisional). Bowel within the hernia becomes ischaemic → gangrene → perforation → peritonitis. Features of intestinal obstruction (vomiting, distension, constipation) may coexist. |
By Mechanism: Other
| Condition | Key Differentiating Features |
|---|---|
| Anastomotic leakage | Post-operative patient (typically day 3–7 after bowel surgery). Sudden deterioration with fever, tachycardia, abdominal pain, peritoneal signs. Peritoneal fluid may be faeculent or bile-stained [1][3]. |
Layer 2: Conditions That Mimic Peritonitis ("Pseudo-peritonitis")
These are critical because they can present with abdominal pain and even some degree of abdominal tenderness/guarding, but the peritoneum itself is NOT the primary site of pathology. Operating on these patients is either unnecessary or harmful.
Retroperitoneal Conditions
Retroperitoneal organs are NOT covered by peritoneum [3], so inflammation of these structures does not directly cause classical peritoneal signs — but the pain may be severe and can be confusing:
| Condition | How to Differentiate |
|---|---|
| Ruptured AAA | Sudden severe abdominal/back pain, hypotension, pulsatile abdominal mass. May mimic peritonitis with abdominal tenderness. CT angiography diagnostic. Ecchymosis signs (Grey-Turner, Cullen) overlap with severe pancreatitis [5]. |
| Acute pancreatitis | Retroperitoneal organ → signs may be less classical initially. Raised amylase/lipase ( > 3× ULN). Pain radiating to back. No free gas on CXR (distinguishes from PPU). |
| Perinephric abscess / pyelonephritis | Flank pain, fever, costovertebral angle tenderness. Pyuria and bacteriuria on urinalysis. CT: perinephric collection. |
| Ureteric colic | Severe colicky loin-to-groin pain. Restless patient (writhing, cannot stay still — opposite to peritonitis where patients lie still). Haematuria on dipstick. CT KUB diagnostic. |
Gynaecological Conditions (Critical DDx in Women of Reproductive Age)
These are extremely important — several of these present almost identically to appendicitis or pelvic peritonitis:
| Condition | How to Differentiate |
|---|---|
| Ruptured ectopic pregnancy | Life-threatening. Amenorrhoea + positive β-hCG + acute lower abdominal pain + haemodynamic instability. Free fluid on FAST USG. Always do a pregnancy test in any woman of reproductive age with acute abdominal pain [2]. |
| Pelvic inflammatory disease (PID) | Bilateral lower abdominal pain, fever, purulent vaginal discharge. Pain worsens during/shortly after menses or with coitus. Cervical motion tenderness ("chandelier sign") on bimanual examination [2]. |
| Tubo-ovarian abscess | Complication of PID. Inflammatory mass in adnexa. High fever, toxic. Can rupture → generalised peritonitis [2]. |
| Ruptured ovarian cyst | Sudden onset of unilateral lower abdominal pain, often during exercise or intercourse. May cause haemoperitoneum. Diagnosis: USG showing free fluid + collapsed cyst [2]. |
| Ovarian / fallopian tube torsion | Sudden severe unilateral pelvic pain, nausea/vomiting. Whirlpool sign on Doppler USG. Adnexal mass with absent blood flow [2]. |
| Endometriosis / Endometrioma | Cyclical pelvic pain, dysmenorrhoea, dyspareunia. Endometrioma rupture can cause chemical peritonitis (chocolate-coloured fluid). |
Golden Rule
Always do a pregnancy test (urine or serum β-hCG) in ANY woman of reproductive age presenting with acute abdominal pain. A ruptured ectopic pregnancy can kill within hours. This is the single most important "don't miss" diagnosis.
Medical Causes ("Non-Surgical Abdomen" Mimicking Peritonitis)
These are conditions where laparotomy is not indicated — operating on these patients causes harm:
| Condition | Why It Mimics Peritonitis | How to Differentiate |
|---|---|---|
| Diabetic ketoacidosis (DKA) | Severe abdominal pain (mechanism: gastric dilatation, mesenteric ischaemia from dehydration, metabolic irritation of peritoneum). Can cause guarding and even board-like rigidity. | Hyperglycaemia, ketonaemia, metabolic acidosis with high anion gap. History of type 1 DM or missed insulin. Kussmaul breathing, fruity breath. ABG diagnostic [4]. |
| Acute MI (especially inferior) | Inferior MI can refer pain to the epigastrium via diaphragmatic irritation (shared innervation T7–T9). | ECG, troponin. Risk factors for IHD. No peritoneal signs on careful examination. |
| Addisonian crisis | Severe abdominal pain, hypotension, vomiting. | Hyperkalaemia, hyponatraemia, hypoglycaemia. History of steroid use/withdrawal. Low random cortisol. |
| Herpes zoster (shingles) | Dermatome distribution of pain may precede the rash by days → can mimic peritonitis of the corresponding abdominal quadrant. | Unilateral, dermatomal. Vesicular rash eventually appears. |
| Acute porphyria | Severe colicky abdominal pain, neuropsychiatric symptoms, dark urine. | Urine porphobilinogen elevated. Young woman. |
| Hypercalcaemia | Abdominal pain, constipation, confusion ("bones, stones, groans, moans"). | Serum calcium elevated. PTH, malignancy workup. |
| Sickle cell crisis | Vaso-occlusive crisis can cause severe abdominal pain mimicking an acute abdomen. | Known sickle cell disease. Blood film, Hb electrophoresis. |
| Familial Mediterranean Fever (FMF) | Recurrent episodes of sterile peritonitis with fever, serositis. | Mediterranean ethnicity. Self-limiting episodes. Responds to colchicine. MEFV gene mutation. |
The 'Medical Abdomen' Trap
DKA is the classic exam trap. A young patient with Type 1 DM presents with severe abdominal pain, vomiting, and abdominal rigidity — the surgical team is called for a "peritonitis." But checking a blood glucose and ABG reveals the diagnosis. Always check glucose and ABG in any patient with unexplained peritonitis. Fever, leucocytosis, acidosis, sepsis of unexplained cause in the elderly [1] could also be a medical abdomen — but equally could be masked surgical pathology, so maintain high suspicion.
Abdominal Wall Pathology
| Condition | How to Differentiate |
|---|---|
| Rectus sheath haematoma | Localized abdominal wall pain and mass. Carnett's sign positive (tenderness increases when patient tenses abdominal wall by lifting head — opposite to intra-abdominal pathology where tenderness decreases with tensing because the tensed muscle "protects" the viscera). History of anticoagulation, trauma, or vigorous coughing. CT diagnostic. |
Extra-abdominal Conditions
| Condition | How to Differentiate |
|---|---|
| Lower lobe pneumonia / basal pleurisy | Diaphragmatic irritation → referred upper abdominal pain. Cough, dyspnoea, pleuritic chest pain. CXR shows consolidation. |
| Testicular torsion | Acute scrotal pain may be referred to the lower abdomen. Always examine the scrotum in a young male with lower abdominal pain. |
Systematic Approach — Decision Diagram
Key Clinical Clues to Narrow the Differential
| Clinical Clue | Points Towards |
|---|---|
| Free gas under diaphragm on erect CXR | Perforated hollow viscus (PPU, perforated bowel) → proceed to laparotomy [3] |
| Bile-stained peritoneal fluid | Biliary perforation / GB injury [1][3] |
| Faeculent peritoneal fluid | Perforated colon [1][3] |
| ↑ Amylase in peritoneal fluid | Pancreatitis or perforated duodenum/small bowel [1][3] |
| ↑ Creatinine in peritoneal fluid | Bladder / urinary tract injury [1] |
| Monomicrobial culture from ascitic fluid | SBP (primary peritonitis) [1][2] |
| Polymicrobial culture from ascitic fluid | Secondary peritonitis (perforation/breach) [1][2] |
| Lymphocyte-predominant ascitic fluid + high ADA | TB peritonitis [1] |
| Turbid PD effluent in a PD patient | CAPD peritonitis [2] |
| Positive β-hCG | Ruptured ectopic pregnancy |
| Cervical motion tenderness | PID |
| High anion gap metabolic acidosis + hyperglycaemia | DKA (medical mimic) |
| Pain out of proportion to examination | Mesenteric ischaemia (early) |
| Dermatomal pain pattern | Herpes zoster |
Special Consideration: Differentiating Diverticulitis from CRC
This is specifically highlighted in the senior notes [2] and worth emphasising:
- Similar clinical features and bowel wall thickening on abdominal CT scan [2]
- Features suggestive of acute diverticulitis include: presence of pericolonic and mesenteric inflammation, involvement of > 10 cm of colon, and absence of enlarged pericolonic lymph nodes on CT [2]
- CRC can only be excluded with colonoscopy after resolution of acute inflammation [2] — you should NEVER do a colonoscopy during the acute episode (risk of perforation)
- In Asian populations, right-sided diverticulitis is significantly more common and is OFTEN confused with acute appendicitis [2]
Special Consideration: DDx of RIF Pain (Appendicitis vs. Everything Else)
Since appendicitis progressing to peritonitis is so common, and RIF pain has a broad differential [2]:
GI: Acute appendicitis, right-sided diverticulitis (more common in Asians), Meckel's diverticulitis, acute ileitis (Yersinia, Campylobacter, Salmonella), Crohn's disease, caecal carcinoma, mesenteric adenitis
O&G: Ruptured ectopic pregnancy, PID, tubo-ovarian abscess, ruptured ovarian cyst, ovarian/fallopian tube torsion, endometriosis [2]
Urological: Ureteric colic, UTI/pyelonephritis
Other: Strangulated inguinal/femoral hernia, psoas abscess
High Yield Summary — Differential Diagnosis of Peritonitis
Layer 1 — Causes of peritonitis (what's the aetiology?):
- Primary: SBP (cirrhosis), CAPD peritonitis (PD patient), TB peritonitis (insidious, laparoscopic biopsy)
- Secondary (most common): Perforation (PPU, appendix, diverticular, CRC, trauma), Inflammation (cholecystitis, appendicitis, pancreatitis, diverticulitis), Ischaemia (mesenteric ischaemia, strangulated hernia), Anastomotic leak
- Tertiary: Persistent despite adequate Rx — opportunistic organisms
Layer 2 — Mimics of peritonitis:
- Medical: DKA (classic trap!), inferior MI, Addisonian crisis, porphyria, herpes zoster, hypercalcaemia, sickle cell, FMF
- Retroperitoneal: Ruptured AAA, pancreatitis, perinephric abscess, ureteric colic
- Gynaecological (always pregnancy test!): Ruptured ectopic, PID, TOA, ovarian cyst rupture, torsion
- Abdominal wall: Rectus sheath haematoma (Carnett's sign)
- Extra-abdominal: Basal pneumonia, testicular torsion
Key distinguishing clues: Free gas = perforation → laparotomy. Bile-stained/faeculent fluid = perforated GI tract. Monomicrobial = primary. Polymicrobial = secondary. Lymphocytic + high ADA = TB. Turbid PD effluent = CAPD peritonitis. Positive β-hCG = ectopic. High AG metabolic acidosis = DKA.
Active Recall - Differential Diagnosis of Peritonitis
1. A cirrhotic patient with ascites develops fever and abdominal pain. Ascitic fluid culture grows 3 different organisms. Is this SBP or secondary peritonitis, and why does the distinction matter?
Show mark scheme
This is secondary peritonitis, not SBP. SBP is monomicrobial; polymicrobial culture suggests a breach in the GI tract (e.g. perforation). The distinction matters because SBP is treated with antibiotics alone (3rd-gen cephalosporin), whereas secondary peritonitis requires surgical intervention. Runyon's criteria can further support secondary peritonitis: protein > 1 g/dL, glucose < 50 mg/dL, LDH > ULN for serum.
2. Name 3 medical (non-surgical) conditions that can mimic peritonitis and explain why DKA is the classic trap.
Show mark scheme
DKA, acute inferior MI, Addisonian crisis (also: porphyria, herpes zoster, hypercalcaemia, sickle cell crisis, FMF). DKA mimics peritonitis because severe metabolic derangement causes gastric dilatation, mesenteric ischaemia from dehydration, and metabolic irritation of the peritoneum, producing genuine abdominal tenderness and even board-like rigidity. Performing a laparotomy on a DKA patient is harmful; checking glucose and ABG reveals the diagnosis.
3. A 28-year-old woman presents with acute RIF pain, fever, and localised peritonitis. List 4 important differential diagnoses and state the single most important bedside test you must not forget.
Show mark scheme
DDx: Acute appendicitis, ruptured ectopic pregnancy, PID/tubo-ovarian abscess, ruptured ovarian cyst, right-sided diverticulitis (more common in Asians), ovarian torsion, ureteric colic. The single most important test is a urine pregnancy test (beta-hCG) to exclude ruptured ectopic pregnancy, which is life-threatening.
4. How do you differentiate TB peritonitis from SBP based on clinical presentation and peritoneal fluid analysis?
Show mark scheme
TB peritonitis: insidious onset, low-grade fever, weight loss, peritoneal signs not florid. Ascitic fluid is LYMPHOCYTE-predominant (vs. neutrophil-predominant in SBP), high protein > 2.5 g/dL, elevated ADA > 39 U/L. AFB smear often negative, culture takes 4-6 weeks and may be falsely negative. Diagnosis often requires laparoscopy and peritoneal biopsy showing caseating granulomata. SBP: acute onset, neutrophil-predominant ascites (PMN >= 250), usually in context of decompensated cirrhosis.
5. What peritoneal fluid findings indicate a perforated GI tract rather than primary peritonitis?
Show mark scheme
Bile-stained fluid (biliary perforation), faeculent fluid (colonic perforation), raised amylase (duodenal/small bowel perforation or pancreatitis), raised creatinine above serum level (bladder perforation). Also: polymicrobial culture, and Runyon's criteria positive (protein > 1 g/dL, glucose < 50 mg/dL, LDH > ULN).
References
[1] Lecture slides: GC 195. Lower and diffuse abdominal pain RLQ problems; pelvic inflammatory disease; peritonitis and abdominal emergencies.pdf (p34–43) [2] Senior notes: felixlai.md (Peritonitis section p738–743; Acute appendicitis DDx p728–729; Diverticulitis DDx p641; CAPD peritonitis p866; SBP p449–450) [3] Senior notes: maxim.md (Section 2.5 Peritonitis; Acute abdomen DDx p44–46; Appendicitis p179; Diverticulitis p194) [4] Senior notes: maxim.md (Acute abdomen DDx — medical causes: DKA, hypercalcaemia, herpes zoster, porphyria, p44) [5] Senior notes: felixlai.md (Ruptured AAA DDx p910–911) [6] Senior notes: felixlai.md (Hinchey classification p637)
Diagnostic Criteria
The diagnosis of peritonitis is fundamentally clinical — you see a sick patient with peritoneal signs and you act. However, to classify the type of peritonitis (primary vs. secondary) and guide the correct treatment pathway (antibiotics alone vs. surgery), you need specific diagnostic criteria for each subtype. Let me walk through each one and explain the logic behind every threshold.
1. Spontaneous Bacterial Peritonitis (SBP)
Diagnosis requires ALL of the following [2]:
| Criterion | Threshold | Rationale |
|---|---|---|
| Ascitic fluid PMN count | ≥ 250 cells/mm³ | PMNs (neutrophils) are the first-responders to bacterial infection. A count ≥ 250 is the validated cut-off that balances sensitivity and specificity for SBP. Why neutrophils specifically? Because they are recruited to the peritoneal cavity within hours of bacterial invasion, whereas lymphocytes predominate in TB or malignancy. |
| Peritoneal fluid culture | Positive | Confirms infection and identifies the organism. Monomicrobial — typically Gram-negative enterics (E. coli, Klebsiella) or Gram-positive cocci (Streptococcus, Staphylococcus) [1][2]. Culture must be inoculated into blood culture bottles at the bedside (not just sent in a plain pot) to improve yield — SBP has low bacterial density in ascitic fluid. |
| Secondary causes excluded | Must rule out surgically treatable source | If you miss a perforation and treat as SBP with antibiotics alone, the patient will die. Runyon's criteria (see below) help distinguish. |
Important Variants of SBP [2]
| Variant | PMN Count | Culture | Significance |
|---|---|---|---|
| Classic SBP | ≥ 250/mm³ | Positive | Treat with antibiotics |
| Culture-negative neutrocytic ascites (CNNA) | ≥ 250/mm³ | Negative | Almost certainly early SBP — treat as SBP anyway. Culture is negative because bacterial density is low. |
| Non-neutrocytic bacterascites (NNBA) | < 250/mm³ | Positive | May resolve spontaneously or progress to SBP. Repeat paracentesis in 48h. Treat only if symptomatic. |
Why is PMN ≥ 250 the threshold for SBP, not ≥ 500?
The lecture slides state neutrophil count > 500/μL as a general peritoneal fluid marker for peritonitis [1]. However, for SBP specifically, the threshold is PMN ≥ 250/mm³ [2] — this lower threshold was established because cirrhotic patients are immunosuppressed and mount a weaker inflammatory response. Waiting for PMN > 500 would miss early SBP and delay life-saving antibiotics. The PMN ≥ 250 cut-off has ~93% sensitivity.
2. CAPD-Associated Peritonitis
Diagnosis requires at least 2 of 3 criteria [2]:
| Criterion | Threshold | Rationale |
|---|---|---|
| Clinical features | Abdominal pain or cloudy effluent ± fever | The turbidity of PD effluent is due to increased WBCs — this is often the first sign noticed by the patient before pain develops. Absence of fever does not exclude peritonitis since the infection can be localised or low-grade [2]. |
| PD fluid cell count | WBC ≥ 100 cells/mm³ with PMN > 50% after dwell time ≥ 2 hours | Note this is LOWER than SBP threshold (100 vs. 250). The cutoff is lower because dextrose in PD solutions provides an excellent growth medium for bacteria — rapid proliferation means even a "low" count indicates significant infection [2]. The 2-hour dwell-time requirement standardises the count (shorter dwells may dilute cells). |
| PD effluent culture | Positive | Gram stain is positive in only ~40% of cases, so a negative Gram stain does NOT exclude infection. Effluent must be cultured (aerobic, anaerobic, AFB, fungal). |
Clinical Pearl
In CAPD peritonitis, do not wait for laboratory confirmation or culture result — start antibiotics as early as possible if the diagnosis is clinically certain, since delayed treatment carries a worse outcome [2]. This is because dextrose-containing PD fluid accelerates bacterial growth, and the peritoneal membrane becomes progressively damaged with ongoing infection, leading to fibrosis and eventual PD failure.
3. Secondary Bacterial Peritonitis (Distinguishing from SBP in a Cirrhotic Patient)
This uses Runyon's criteria — at least 2 of 3 in the ascitic fluid [2]:
| Criterion | Threshold | Why This Distinguishes Secondary from Primary |
|---|---|---|
| Total protein | > 1 g/dL (10 g/L) | In SBP, ascitic fluid protein is low (poor opsonic activity — this is WHY they get SBP in the first place). In secondary peritonitis, the perforation allows protein-rich intestinal/serum contents to flood the peritoneal cavity → high protein. |
| Glucose | < 50 mg/dL (2.8 mmol/L) | Neutrophils consume large amounts of glucose [2]. Secondary peritonitis has a much higher neutrophil and bacterial burden than SBP → more glucose consumed → lower glucose. In SBP, the ascitic fluid neutrophil count is lower (by comparison) and glucose remains relatively preserved. |
| LDH | > Upper limit of normal for serum | LDH is released from dying cells. In secondary peritonitis, there is massive tissue destruction (from perforation, ischaemia) → ↑↑↑ LDH [2]. In SBP, LDH is only mildly elevated. |
Additional clues for secondary peritonitis (not part of Runyon's criteria but highly useful):
- Polymicrobial culture (vs. monomicrobial in SBP) [1][2]
- Amylase level ↑ — suggests pancreatitis or gut perforation (every segment of gut except gallbladder leaks amylase into fluid when it perforates [2])
- Bilirubin level ↑ — suggests perforation of gallbladder into peritoneum (measured if fluid is dark orange or brown [2])
Summary Comparison Table: SBP vs. Secondary Peritonitis Ascitic Fluid
| Parameter | SBP | Secondary Peritonitis |
|---|---|---|
| Organisms | Monomicrobial | Polymicrobial |
| PMN count | ≥ 250/mm³ | Often much higher |
| Protein | ↓ (Low) — reflects poor opsonic activity [2] | ↑ (High) — protein-rich GI contents leak in |
| Glucose | ↑ (Relatively preserved) — fewer neutrophils consuming it [2] | ↓ (Low < 50 mg/dL) — massive neutrophil/bacterial consumption |
| LDH | ↑ (Mildly elevated) [2] | ↑↑↑ (Markedly elevated) — tissue destruction [2] |
| Amylase | Normal | ↑↑↑ if gut perforation or pancreatitis [1][2] |
| Bilirubin | Normal | ↑ if GB perforation [2] |
Exam Pearl: The Logic Behind SBP vs. Secondary
Think of it this way: SBP = bacteria are "tourists" that translocated into a fluid with poor defences. There aren't many of them, they don't destroy tissue, and the fluid hasn't been contaminated by GI contents → monomicrobial, low protein, preserved glucose, mildly raised LDH. Secondary peritonitis = a pipe has burst — GI contents (protein, bacteria, amylase, bile) flood the peritoneal cavity, massive neutrophil recruitment consumes glucose, tissue dies releasing LDH → polymicrobial, high protein, low glucose, very high LDH.
Investigation Modalities
Investigations for peritonitis serve three purposes: (1) confirm peritonitis, (2) identify the cause, and (3) assess severity/guide resuscitation. I'll organise these by modality.
A. Bedside Tests
| Investigation | Key Findings | Rationale / Interpretation |
|---|---|---|
| Urinalysis | Sterile pyuria (in diverticulitis/appendicitis adjacent to ureter), haematuria (ureteric colic), nitrites/leucocytes (UTI) | Sterile pyuria can occur from peritoneal inflammation irritating the adjacent ureter — don't be fooled into diagnosing UTI [1]. |
| Pregnancy test (urine β-hCG) | Positive → ruptured ectopic pregnancy | Must be done in ANY woman of reproductive age with acute abdominal pain [1][3]. A positive test completely changes your differential and management. |
| ECG | ST changes, arrhythmias | Rule out acute MI [3] — especially inferior MI which can mimic upper abdominal peritonitis via diaphragmatic irritation. Also check for AF (risk factor for mesenteric ischaemia). |
B. Blood Tests
Blood tests: CBC, LRFT, amylase, ABG, clotting, T&S [3]
| Investigation | Key Findings | Rationale / Why Order It |
|---|---|---|
| CBC with differentials | Leukocytosis (raised WCC with neutrophilia) [2] | The systemic inflammatory response to peritoneal infection drives bone marrow neutrophil release. In the elderly, leucocytosis may be the only clue to peritonitis when peritoneal signs are mild [1]. A left shift (bandaemia — immature neutrophils) suggests severe/acute infection. Leucopaenia is ominous (marrow exhaustion in overwhelming sepsis). |
| LFT | Deranged bilirubin, ALT, AST, ALP, GGT | Sepsis can lead to deranged liver function [2] — "septic liver" (cholestatic pattern from intrahepatic bile duct dysfunction due to endotoxins). Also baseline assessment in cirrhotic patients. Raised bilirubin + ALP may point to cholangitis as the cause. |
| RFT (renal function) | Raised urea and creatinine | Hypovolaemia leads to acute kidney injury [2] — pre-renal AKI from third-space losses. Also baseline for patients on nephrotoxic antibiotics (aminoglycosides). Raised urea out of proportion to creatinine suggests upper GI bleeding (urea from digested blood). |
| Serum amylase / lipase | Elevated ( > 3× ULN in pancreatitis) [2] | Pancreatitis as the cause of peritonitis. Also mildly raised in bowel ischaemia, perforated ulcer, and small bowel obstruction. Lipase is more specific than amylase for pancreatic pathology. |
| Serum protein / albumin | Hypoalbuminaemia | Massive protein loss into the peritoneal cavity (third-spacing). Also baseline for SAAG calculation in ascitic patients. |
| ABG with lactate | Metabolic acidosis, raised lactate | Raised lactate indicates tissue hypoperfusion (shock) or bowel ischaemia — a lactate > 2 mmol/L in the context of abdominal pain is a red flag for mesenteric ischaemia. Metabolic acidosis also seen in DKA (the medical mimic) [3]. |
| Clotting profile (PT, APTT) | Prolonged PT/APTT | Obtain baseline before endoscopic and surgical procedures [2]. Cirrhotic patients have impaired synthetic function → coagulopathy. DIC can develop in severe sepsis. |
| Type and screen | Blood group and antibody screen | Obtain baseline before endoscopic and surgical procedures [2]. In case emergency surgery is needed, blood must be available for transfusion. |
| CRP | Elevated | Non-specific inflammatory marker. Useful for monitoring treatment response. A CRP that fails to fall after treatment suggests uncontrolled source (abscess, ongoing leak). |
| Blood glucose | Hyperglycaemia (DKA, stress response) or hypoglycaemia (sepsis, liver failure) | Excludes DKA as a mimic. In CAPD peritonitis, infection will induce hyperglycaemia [2]. |
| Blood cultures | Organism identification | Must be taken before starting antibiotics. Aerobic + anaerobic bottles. Positive in ~30–50% of SBP. |
C. Peritoneal Fluid Analysis
This is the cornerstone investigation for primary peritonitis (SBP and CAPD). For secondary peritonitis, peritoneal fluid is often obtained intra-operatively rather than pre-operatively.
Ascitic fluid analysis by paracentesis [2]
Peritoneal fluid analysis [1]:
| Component | What to Look For | Interpretation |
|---|---|---|
| Character | Serous, blood-stained, purulent, bile-stained, faeculent [1] | Serous = SBP or early infection. Purulent = established bacterial peritonitis. Bile-stained = perforated GB or biliary injury. Faeculent = perforated bowel. Blood-stained = trauma, malignancy, haemorrhagic pancreatitis. ↑ Amylase / bile-stained / faeculent indicates perforated GI tract [3]. |
| Cell count and differentials | Neutrophil count > 500/μL [1]; PMN ≥ 250/mm³ for SBP [2]; WBC ≥ 100/mm³ with PMN > 50% for CAPD [2] | PMN ≥ 250 cells/mm³ should be started on empirical therapy while awaiting culture results [2]. Lymphocyte predominance → TB peritonitis or malignancy. |
| Gram stain | Bacterial morphology | Quick but low sensitivity (~25% in SBP because bacterial density is low). Useful if positive (e.g., Gram-positive cocci in chains → Streptococcus). |
| Cultures | Aerobic, anaerobic, AFB, fungal [1] | Definitive identification. Inoculate into blood culture bottles at bedside. Monomicrobial = SBP; polymicrobial = secondary peritonitis. AFB culture takes 4–6 weeks and could be falsely negative [1]. |
| Albumin level | Calculate SAAG | Serum-ascites albumin gradient (SAAG) = serum albumin − ascitic albumin (difference but NOT ratio [2]). SAAG > 1.1 g/dL = portal hypertension → SBP likely. SAAG < 1.1 g/dL = NO portal hypertension → think TB, malignancy, nephrotic syndrome [2]. |
| Protein level | High vs. low | ↑ Protein in secondary bacterial peritonitis; ↓ Protein in SBP [2]. Low protein in SBP reflects low opsonin activity (molecules that facilitate phagocytosis by macrophages) and patients are more prone to SBP [2]. |
| Glucose | High vs. low | ↑ Glucose in SBP; ↓ Glucose ( < 50 mg/dL) in secondary peritonitis [2]. Why? Neutrophils consume large amounts of glucose — secondary peritonitis has far more neutrophils → more consumption [2]. |
| LDH | Mildly vs. markedly elevated | ↑ LDH in SBP; ↑↑↑ LDH in secondary peritonitis [2]. Reflects degree of tissue destruction. |
| Amylase | Elevated | ↑ Amylase suggests pancreatitis or gut perforation — every segment of gut except gallbladder leaks amylase into fluid when it perforates [2]. |
| Bilirubin | Elevated | ↑ Bilirubin suggests perforation of gallbladder into peritoneum — measured if the ascitic fluid is dark orange or brown [2]. |
| Creatinine | Higher than serum | Ascitic creatinine > serum creatinine → bladder perforation / urinary leak [1]. |
| ADA (Adenosine deaminase) | Elevated ( > 39 U/L) | ↑ ADA level in tuberculosis infection [2]. ADA is a purine-degrading enzyme necessary for maturation and differentiation of lymphoid cells [2] — elevated because TB triggers a vigorous lymphocytic response. |
| Cytology | Malignant cells | Positive in < 10% of malignant ascites (poor sensitivity, in contrast with pleural fluid cytology with 70% chance of detection in malignant pleural effusion) [2]. |
D. Imaging
Imaging: ECG, Erect CXR, erect/supine AXR, USG, CT A+P [3]
| Modality | Key Findings | Rationale / When to Use |
|---|---|---|
| Erect CXR | Free gas (pneumoperitoneum) under the diaphragm | The single most important initial imaging investigation in suspected perforated viscus. Free gas = hollow viscus perforation (PPU, perforated bowel). Look for free gas under diaphragm [2]. Also detects basal pneumonia (mimic), pleural effusion (reactive). As little as 1 mL of free gas can be detected on erect CXR. Must be erect for ≥ 10 minutes before shooting to allow gas to rise. |
| Erect and supine AXR | Dilated bowels in intestinal obstruction [2]; air-fluid levels; radio-opaque calculi; loss of psoas shadow (retroperitoneal pathology) | Supine AXR shows bowel gas pattern and distribution. Erect AXR shows air-fluid levels. Dilated small bowel ( > 3 cm) or large bowel ( > 6 cm, caecum > 9 cm) suggests obstruction. Free gas may also be seen on supine AXR as Rigler's sign (gas on both sides of bowel wall). |
| USG abdomen | Free fluid (Morison's pouch), gallstones, thickened GB wall, appendiceal diameter > 6 mm, adnexal pathology, abscess | Evaluation of acute cholecystitis, appendicitis and gynaecological infections [2]. First-line for RUQ pain (biliary) and suprapubic pain (gynae/urological) [3]. FAST scan in trauma to detect haemoperitoneum. Can detect as little as 200 mL of free fluid in Morison's pouch. |
| CT abdomen + pelvis (with IV contrast) | Free gas, free fluid, bowel wall thickening, fat stranding, abscess collections, mesenteric vessel occlusion, appendiceal inflammation | Gold standard for secondary peritonitis — identifies the source. CT is particularly useful for diverticulitis (CT scan helps to confirm diagnosis and assess severity [1]), appendicitis, and detecting intra-abdominal abscesses. Can guide percutaneous drainage. CT angiography for mesenteric ischaemia. CT with IV contrast is first-line for RLQ and LLQ pain [3]. |
| Colonoscopy | Mucosal pathology, stricture, tumour, colitis | Look for bowel ischaemia [2]. Used after resolution of acute inflammation to exclude CRC or IBD. AVOID endoscopy for acute abdomen — sealed-off perforation may open by gas insufflation during endoscopy [3]. |
| Diagnostic laparoscopy | Direct visualisation of peritoneal cavity | Used when diagnosis remains uncertain despite imaging. Diagnosis of TB peritonitis is often made by laparoscopy and biopsy of peritoneum [1]. Also therapeutic — can perform lavage, take biopsies, and even definitive surgery (appendicectomy, PPU repair). |
When NOT to Image — Go Straight to Theatre
Proceed to exploratory laparotomy if free gas / florid peritoneal signs [3]. If a patient has generalised peritonitis with haemodynamic instability, spending hours on CT delays definitive treatment. The surgical dictum: "If the clinical picture says operate, operate. CT is for when you're not sure."
Diagnostic Algorithm
The clinical approach follows a logical sequence: resuscitate → clinical assessment → bedside tests → blood tests → imaging → peritoneal fluid analysis → decide: operate or treat medically.
Step-by-Step Walkthrough
Step 1 — Resuscitate and Assess Simultaneously
You never wait for investigation results before starting resuscitation. A patient with peritonitis is losing fluid into the peritoneal cavity (third-spacing) and may be septic. IV fluid replacement, nasogastric tube, urinary catheter, oxygen [1] should be started immediately while you take the history, examine, and order tests.
Step 2 — Bedside Tests
- Urinalysis — excludes UTI, detects haematuria (ureteric colic), sterile pyuria (peri-ureteric inflammation) [1]
- Pregnancy test — mandatory in reproductive-age women [1]
- ECG — excludes inferior MI [3]
- Blood glucose — excludes DKA
Step 3 — Blood Tests
Blood count, renal and liver function, amylase, clotting profile, arterial blood gas, type and screen [1][3]. Blood cultures before antibiotics. The ABG with lactate is critical — a raised lactate > 2 mmol/L suggests tissue hypoperfusion or bowel ischaemia.
Step 4 — Initial Imaging (Erect CXR + AXR)
Erect CXR is the first imaging — looking for free gas. If present, you have a perforated viscus and may proceed directly to surgery. Erect and supine AXR — look for dilated bowels (obstruction), air-fluid levels, calculi.
Step 5 — Decision Point: Free Gas?
If free gas + florid peritoneal signs → proceed to exploratory laparotomy [3]. Do NOT delay with further imaging in an unstable patient.
If no free gas but peritoneal signs present → further investigation needed to identify the source.
Step 6 — Further Imaging / Fluid Analysis
- Cirrhotic with ascites: Diagnostic paracentesis → ascitic fluid analysis (cell count, culture, protein, glucose, LDH, albumin for SAAG, ± amylase/bilirubin/ADA/cytology) [2]
- PD patient: Collect PD effluent for cell count + culture [2]
- All others: USG abdomen for RUQ/pelvic pathology; CT abdomen + pelvis with contrast for most other scenarios [1][2][3]
- If diagnosis remains elusive: diagnostic laparoscopy [1]
Step 7 — Classify and Treat
Based on the above, classify as primary (SBP/CAPD/TB) vs. secondary (surgical source) vs. tertiary (persistent/opportunistic) and institute appropriate management.
Investigation Selection by Site of Pain
This is a practical guide from the clinical approach [3]:
| Site of Pain | Imaging of Choice |
|---|---|
| RUQ | USG (biliary pathology — cholecystitis, choledocholithiasis) |
| LUQ | CT (splenic pathology, pancreatitis) |
| RLQ | CT with IV contrast (appendicitis, right-sided diverticulitis, Crohn's) |
| LLQ | CT with IV contrast (left-sided diverticulitis, sigmoid pathology) |
| Suprapubic | USG (TAS or TVS) (gynaecological, bladder pathology) |
| Diffuse | Erect CXR first, then CT abdomen + pelvis if no free gas [3] |
Special Investigation Scenarios
TB Peritonitis
Peritoneal fluid: AFB smear often negative, culture would take 4–6 weeks (could be falsely negative) [1]. This makes diagnosis challenging. Key investigations:
- Ascitic fluid: Lymphocyte-predominant, high protein ( > 2.5 g/dL), ↑ ADA level [2], low SAAG ( < 1.1 g/dL)
- Diagnosis often made by laparoscopy and biopsy of peritoneum [1] — looking for caseating granulomata and "millet seed" tubercles studding the peritoneal surface
- Interferon-gamma release assay (IGRA) / Mantoux can support but not confirm peritoneal TB
- PCR for M. tuberculosis DNA in ascitic fluid — increasing role
Diverticulitis-Related Peritonitis
CT scan helps to confirm diagnosis and assess the severity [1]. CT findings include:
- Colonic diverticula, localised bowel wall thickening ( > 4 mm), pericolonic fat stranding
- Abscess (fluid collection with air/debris), free gas (perforation), fistula tracks
- Hinchey classification applied based on CT findings to guide management [6]
High Yield Summary — Diagnostics
Diagnostic Criteria:
- SBP: PMN ≥ 250/mm³ + positive culture + secondary causes excluded. SAAG > 1.1 = portal hypertension → SBP likely. Monomicrobial.
- CAPD peritonitis: Clinical features (pain/cloudy effluent) + WBC ≥ 100/mm³ with PMN > 50% (dwell ≥ 2h) + positive culture. Lower threshold than SBP due to dextrose-enhanced bacterial growth.
- Secondary peritonitis (Runyon's): ≥ 2 of: protein > 1 g/dL, glucose < 50 mg/dL, LDH > ULN. Polymicrobial. Needs surgery.
Key Investigations:
- Bedside: Urinalysis, pregnancy test, ECG, glucose
- Bloods: CBC, LRFT, amylase, ABG/lactate, clotting, T&S, blood cultures
- Imaging: Erect CXR (free gas!) + AXR → USG or CT A+P
- Peritoneal fluid: Character, cell count, Gram stain, cultures (aerobic/anaerobic/AFB/fungal), albumin (SAAG), protein, glucose, LDH, amylase, bilirubin, creatinine, ADA, cytology
Decision Rule: Free gas on erect CXR + florid peritoneal signs → exploratory laparotomy (do NOT delay with further imaging).
SBP vs. Secondary: SBP = monomicrobial, low protein, preserved glucose, mildly raised LDH. Secondary = polymicrobial, high protein, low glucose, very high LDH.
Active Recall - Diagnosis and Investigations of Peritonitis
1. State the diagnostic criteria for SBP and explain why the PMN threshold is 250/mm3 rather than 500/mm3.
Show mark scheme
SBP requires ALL of: (1) Ascitic fluid PMN >= 250 cells/mm3, (2) Positive peritoneal fluid culture, (3) Secondary causes excluded. The threshold is lower at 250 (not 500) because cirrhotic patients are immunosuppressed and mount a weaker inflammatory response; waiting for 500 would miss early SBP and delay life-saving antibiotics. The 250 cut-off has ~93% sensitivity.
2. Why is the WBC threshold for CAPD peritonitis (100/mm3) lower than for SBP (250/mm3)?
Show mark scheme
PD fluid contains dextrose which provides an excellent growth medium for bacteria, leading to rapid bacterial proliferation. Even a lower WBC count indicates significant infection in this environment. Without antibiotic therapy, bacteria multiply quickly in the dextrose-rich PD fluid, so a lower threshold ensures early detection.
3. A cirrhotic patient with ascites develops peritonitis. Ascitic fluid shows: protein 1.5 g/dL, glucose 30 mg/dL, LDH above ULN, polymicrobial culture. Is this SBP or secondary peritonitis? Justify using Runyon's criteria.
Show mark scheme
This is secondary peritonitis. Runyon's criteria met (all 3 of 3, need >= 2): protein > 1 g/dL (1.5), glucose < 50 mg/dL (30), LDH > ULN. Additionally polymicrobial culture confirms secondary peritonitis. This patient needs CT to identify source and likely surgical intervention, not just antibiotics.
4. What does a SAAG > 1.1 g/dL indicate and how is it calculated? What does SAAG < 1.1 indicate?
Show mark scheme
SAAG = Serum albumin minus ascitic fluid albumin (difference, NOT ratio). SAAG > 1.1 g/dL indicates portal hypertension (cirrhosis, heart failure, Budd-Chiari) and thus SBP is a likely cause of peritonitis. SAAG < 1.1 indicates no portal hypertension - think TB peritonitis, malignancy, nephrotic syndrome, pancreatic ascites.
5. When should you proceed directly to exploratory laparotomy without further imaging in suspected peritonitis?
Show mark scheme
When there is free gas under the diaphragm on erect CXR AND/OR florid peritoneal signs (diffuse tenderness, guarding, rigidity, rebound) with haemodynamic instability. Do not delay with CT or other imaging in an unstable patient with clear signs of perforated viscus and generalised peritonitis.
6. List 3 ascitic fluid findings that specifically point to a perforated GI tract rather than SBP.
Show mark scheme
(1) Raised amylase - every segment of gut except gallbladder leaks amylase when perforated; also pancreatitis. (2) Bile-stained fluid - suggests gallbladder/biliary perforation. (3) Faeculent fluid - perforated bowel (colonic). Also: raised bilirubin (dark orange/brown fluid = GB perforation), polymicrobial culture, Runyon's criteria positive (high protein, low glucose, high LDH).
References
[1] Lecture slides: GC 195. Lower and diffuse abdominal pain RLQ problems; pelvic inflammatory disease; peritonitis and abdominal emergencies.pdf (p12, p19, p34–43) [2] Senior notes: felixlai.md (Peritonitis diagnosis section p740–742; Ascitic fluid analysis p740–741, p448–449; SBP diagnosis and variants p449–450; CAPD peritonitis diagnostic criteria p866–867; Case study p743) [3] Senior notes: maxim.md (Section 2.5 Peritonitis p46; Acute abdomen investigations p45; Imaging by site of pain p45) [6] Senior notes: felixlai.md (Hinchey classification p637)
Management Overview
The management of peritonitis is fundamentally dictated by one question: is there a surgically treatable source? If yes (secondary peritonitis), surgery is the definitive treatment. If no (primary peritonitis), the treatment is medical. Everything else — resuscitation, antibiotics, supportive care — is built around this central decision.
Think of it as three simultaneous streams happening in parallel:
- Resuscitate the patient (they are losing fluid and becoming septic)
- Treat the infection (antibiotics)
- Eliminate the source (surgery if secondary; antibiotics alone if primary)
Management Algorithm
Immediate Resuscitation (All Types of Peritonitis)
Before you even know the cause, start resuscitation. The patient is dying from two things simultaneously: hypovolaemia (third-space losses) and sepsis (bacterial/endotoxin absorption). Both must be addressed urgently.
Supportive Measures
These are directly from the lecture slides for acute secondary bacterial peritonitis — treatment [1]:
| Measure | Rationale |
|---|---|
| IV fluid replacement | The inflamed peritoneum (1.7 m² surface area) leaks protein-rich fluid into the peritoneal cavity → intravascular depletion. Crystalloids (normal saline, Hartmann's solution) are first-line. Colloids (albumin) may be needed in cirrhotic patients with SBP to prevent hepatorenal syndrome. Target: adequate urine output ( > 0.5 mL/kg/hr), normalisation of lactate, MAP > 65 mmHg. [1] |
| Nasogastric tube (NGT) | Peritonitis causes paralytic ileus → gastric and intestinal contents accumulate → risk of vomiting and aspiration, especially during induction of anaesthesia. The NGT decompresses the stomach ("drip and suck") [1][2]. Placed on free drainage with 4-hourly aspiration. |
| Urinary catheter | Accurate measurement of urine output is essential for monitoring fluid resuscitation and detecting early AKI. Target UOP > 0.5 mL/kg/hr [1]. |
| Oxygen | Peritonitis patients are hypoxic from multiple mechanisms: (1) abdominal distension pushing the diaphragm up → basal atelectasis, (2) tachypnoea from metabolic acidosis, (3) sepsis-related acute lung injury. Supplemental O₂ maintains tissue oxygenation [1]. |
| Pain relief | Adequate analgesia is essential and humane. IV opioids (morphine, fentanyl) are appropriate. Pain relief should not be withheld for fear of "masking signs" — this is an outdated concept. However, reassess after analgesia: if peritoneal signs persist despite good pain relief, they are real [1]. |
| Broad-spectrum antibiotics | Started empirically before culture results are available. The choice depends on whether peritonitis is primary or secondary (see below). Do not wait for laboratory confirmation or culture result since delayed treatment carries a worse outcome [2]. |
| Close monitoring for change of condition | Serial vital signs (HR, BP, RR, temp, SpO₂), urine output, abdominal examination. Look for signs of deterioration: rising HR, falling BP, worsening tenderness, increasing distension [1]. |
The 'Drip and Suck' Principle
"Drip" = IV fluids to replace the third-space losses. "Suck" = NGT to decompress the stomach and prevent aspiration. This combination is the foundation of supportive care in any patient with peritonitis or intestinal obstruction. It buys time while you work out the definitive plan.
Treatment by Type of Peritonitis
1. Primary Peritonitis — Medical Management
Primary peritonitis has no surgical source — there is nothing to cut out. Treatment is antibiotics ± addressing the underlying predisposition.
A. Spontaneous Bacterial Peritonitis (SBP)
Indications for antibiotic therapy (any of the following) [2]:
- Fever > 37.8°C
- Abdominal pain or tenderness
- Altered mental status
- Ascitic fluid PMN count ≥ 250 cells/mm³
Empirical Antibiotic Choice:
3rd generation cephalosporin should be used [2]:
- Cefotaxime 2 g IV Q8h OR
- Ceftriaxone 2 g IV daily
Why 3rd-gen cephalosporins? "Ceph" = cephalosporin (β-lactam antibiotic that inhibits cell wall synthesis). The "3rd generation" designation means it has expanded Gram-negative coverage (especially E. coli, Klebsiella) while retaining some Gram-positive activity. These are the predominant organisms in SBP. They also have good peritoneal penetration.
Duration: 5 days — treatment can be stopped when PMN < 250 cells/mm³ [2]. A repeat paracentesis at 48 hours is recommended to confirm response (expect ≥ 25% decrease in PMN count).
Adjunctive therapy — IV Albumin:
- IV albumin (1.5 g/kg on day 1, then 1 g/kg on day 3) is given alongside antibiotics in SBP patients with renal impairment (creatinine > 88 μmol/L or BUN > 10.7 mmol/L) or bilirubin > 68 μmol/L to prevent hepatorenal syndrome.
- Why? Albumin expands intravascular volume and prevents the renal vasoconstriction that occurs during SBP due to splanchnic arterial vasodilation and cytokine-mediated renal injury. Reduces mortality from ~30% to ~10%.
Prevention of Recurrence (Secondary Prophylaxis):
Fluoroquinolones for lifelong selective intestinal decontamination (unless patient receives liver transplant with normalisation of liver function) [2]:
- Norfloxacin 400 mg PO daily OR
- Levofloxacin 250 mg PO daily
Why fluoroquinolones work for prophylaxis [2]:
- Incomplete absorption by gut leading to high concentration in the intestinal lumen
- High activity against Gram-negative bacilli (the organisms causing SBP)
- Low bacterial resistance
- Fewer side effects
The mechanism is "selective intestinal decontamination" — the fluoroquinolone stays in the gut lumen at high concentrations, killing the Gram-negative bacteria that would otherwise translocate across the gut wall to cause SBP, while preserving anaerobic flora (which actually prevents colonisation by pathogenic organisms).
Primary Prophylaxis (preventing first episode of SBP):
- Indicated in cirrhotic patients with ascitic fluid protein < 1.5 g/dL (low opsonic activity → high risk)
- Same agents: Norfloxacin or Levofloxacin
- Also indicated short-term (7 days) in any cirrhotic patient with acute GI bleeding (high risk of SBP during variceal bleed)
B. CAPD-Associated Peritonitis
As early as possible if the diagnosis of peritonitis is certain clinically — do not wait for laboratory confirmation or culture result since delayed treatment carries a worse outcome [2].
Empirical Antibiotic Regimen (Intraperitoneal — IP):
1st generation cephalosporin + aminoglycoside [2]:
- Gram-positive cover: 1st generation cephalosporin (cefazolin) — covers Staphylococcal species (the most common cause from skin flora around the catheter)
- Gram-negative cover: Aminoglycoside (e.g., gentamicin) OR 3rd or 4th generation cephalosporin (ceftazidime / cefepime)
General rules [2]:
- Use less broad-spectrum drugs — start narrow and escalate if needed
- Avoid vancomycin as initial treatment — to minimise emergence of resistant strains (VRE — vancomycin-resistant enterococci). Reserve vancomycin for MRSA or when 1st-gen cephalosporin fails.
Why IP (intraperitoneal) route? Because the infection is IN the peritoneal cavity. IP antibiotics achieve much higher local concentrations than IV antibiotics. The drug is dissolved in the PD fluid and instilled directly into the peritoneum during a regular exchange.
Modification [2]:
- Replacing aminoglycoside with 3rd generation cephalosporin is advocated to preserve residual renal function and avoid ototoxicity — aminoglycosides are nephrotoxic and ototoxic; PD patients often have some residual renal function that is important to preserve.
Continuation of PD:
- Continuous peritoneal dialysis should be continued unless peritonitis is refractory to treatment [2]. Stopping PD would mean the patient loses their dialysis modality and needs temporary haemodialysis.
Adjust According to Culture:
- Adjust antibiotic therapy according to culture result and sensitivity [2] once results are available (usually 48–72 hours).
Success Rate:
- Success rate > 90% [2]
- Lower success rate for Gram-negative organisms [2]
- Much lower success rate for fungal peritonitis [2]
Indications for Tenckhoff Catheter Removal [2]:
| Indication | Rationale |
|---|---|
| Refractory peritonitis with failure of effluent to clear up after 5 days of antimicrobial treatment | Persistent infection despite adequate antibiotics means the catheter itself is acting as a nidus (biofilm on catheter surface harbours bacteria inaccessible to antibiotics). Switch to temporary haemodialysis. |
| Peritonitis due to hydrophilic Gram-negative rods (e.g., Pseudomonas sp.) | These organisms form tenacious biofilms on catheters. Antimicrobial therapy is usually not sufficient and PD catheter removal is required to ensure complete eradication of infection [2]. |
| Fungal infection (e.g., yeast / Candida) | Fungal peritonitis has very poor response to antifungals alone when the catheter remains in situ — biofilm formation is a major issue. Remove catheter + systemic antifungal (fluconazole or echinocandin). |
Post-removal plan [2]:
- Tenckhoff catheter should be removed and reinserted for peritoneal dialysis few weeks later after total subsidence of peritonitis signs and symptoms
- Patient maintained on haemodialysis in the interim
Blood Glucose Control in CAPD Peritonitis
Infection will induce hyperglycaemia (stress response → counter-regulatory hormones → insulin resistance). However, nausea and anorexia of peritonitis will lead to malnutrition and hence hypoglycaemia in patients receiving hypoglycaemic agents or insulin [2]. This dual risk means blood glucose must be monitored closely and hypoglycaemic medications adjusted.
C. Tuberculous Peritonitis
- Standard anti-TB therapy: RIPE regimen (Rifampicin, Isoniazid, Pyrazinamide, Ethambutol) for 2 months intensive phase, followed by Rifampicin + Isoniazid for 4–7 months continuation phase (total 6–9 months)
- Consider adding pyridoxine (vitamin B6) to prevent isoniazid-induced peripheral neuropathy
- Surgery is NOT indicated unless complications arise (e.g., bowel obstruction from TB strictures, perforation)
- Response to treatment is monitored clinically (resolution of symptoms, weight gain) and by serial ascitic fluid analysis
2. Secondary Peritonitis — Surgical Management
Accounts for most peritonitis [1]. The fundamental principle is source control — you must eliminate the source of contamination. Antibiotics alone are insufficient because bacteria will continue to pour into the peritoneal cavity from the uncontrolled source.
Surgical correction of underlying pathology — laparotomy if surgically treatable source of infection is documented [2]
Indications for Emergency / Urgent Surgery
From the lecture slides on indications for urgent surgery [7]:
- Incarcerated, strangulated hernia
- Suspected or proven strangulation
- Peritonitis
- Pneumoperitoneum
- Pneumatosis cystoides intestinalis (gas within bowel wall — indicates transmural ischaemia/necrosis)
- Close-loop obstruction
- Volvulus with peritoneal signs
The overarching rule: proceed to exploratory laparotomy if free gas / florid peritoneal signs [3]
Surgical Approach
The management has two components, performed simultaneously or sequentially:
Component 1: Source Control (stop the contamination)
| Source | Surgical Procedure |
|---|---|
| PPU (perforated peptic ulcer) | PPU repair — omental patch (Graham patch) closure of perforation ± definitive ulcer surgery [1] |
| Perforated appendicitis | Appendicectomy — laparoscopic preferred if feasible [1] |
| Cholecystitis / GB perforation | Cholecystectomy [1] — laparoscopic if inflammation permits, otherwise open |
| Perforated diverticular disease | Bowel resection [1] — approach depends on Hinchey staging (see below) |
| Perforated bowel (other causes) | Resection of affected segment ± anastomosis or stoma |
| Ischaemic bowel | Resection of non-viable bowel ± revascularisation (embolectomy / bypass for mesenteric ischaemia) |
| Anastomotic leak | Re-exploration, washout, defunctioning stoma ± re-do anastomosis |
Component 2: Peritoneal Toilet (clean up the contamination)
- Thorough peritoneal lavage with warm normal saline (litres)
- Removal of fibrinous debris, pus, faecal matter
- Ensure all quadrants and paracolic gutters are irrigated
- Drains may be placed in dependent areas (pelvis, subphrenic spaces) if ongoing contamination is expected
Operative Approaches
Laparoscopic surgery / Laparotomy [1]:
| Approach | Indications | Advantages |
|---|---|---|
| Laparoscopic | Haemodynamically stable, localised pathology (e.g., perforated appendicitis, PPU in young patient) | Less wound infection, less post-operative pain, faster recovery, better cosmesis |
| Open laparotomy | Haemodynamically unstable, generalised peritonitis, gross sepsis, unclear source, multiple adhesions | Better access for thorough washout, ability to assess entire bowel, safer in unstable patients |
Non-Operative Source Control
Drainage — percutaneous drainage of abdominal abscess [1]:
For localised secondary peritonitis (contained abscess), percutaneous drainage under imaging guidance (CT or USG) can achieve source control without major surgery:
- Indications: Well-defined, unilocular abscess accessible to percutaneous route; patient stable
- Technique: CT-guided or USG-guided catheter placement into the abscess cavity; leave drain in situ until output minimal and clinical improvement
- When it fails: If the abscess does not resolve, or the patient deteriorates, proceed to surgery
This is particularly relevant in diverticular disease [3][6]:
| Hinchey Stage | Description | Treatment |
|---|---|---|
| I | Localised pericolic abscess | IV antibiotics +/- drainage [3] |
| II | Distant abscess (retroperitoneal/pelvic) | IV antibiotics + drainage [3] — CT-guided percutaneous drainage (5 cm as cut-off: likely resolve by antibiotics vs. require intervention [3]) |
| III | Generalised suppurative peritonitis (abscess ruptured, bowel intact) | Hartmann's operation / One-stage resection [3] — Mortality 25% |
| IV | Faecal peritonitis (bowel wall perforation) | Hartmann's operation [3] — Mortality 50% |
Hartmann's procedure: Resection of the affected sigmoid colon with creation of an end-colostomy (the proximal end is brought out as a stoma) and closure of the distal rectal stump. Why? In the presence of gross faecal contamination, performing a primary anastomosis (joining the two bowel ends together) carries a very high risk of anastomotic leak because the tissues are inflamed, oedematous, and contaminated. The stoma is reversed electively months later once the patient has recovered.
Antibiotic Therapy for Secondary Peritonitis
Broad-spectrum antibiotics [1] — must cover Gram-negatives, Gram-positives, AND anaerobes (reflecting the polymicrobial nature of secondary peritonitis):
| Regimen | Coverage | Notes |
|---|---|---|
| Augmentin (amoxicillin-clavulanate) ± Metronidazole | Gram-pos, Gram-neg, anaerobes | Augmentin actually has anaerobic coverage [8], but metronidazole is often added for severe intra-abdominal sepsis (enhanced anti-anaerobic activity) |
| Cefuroxime + Metronidazole | Gram-pos, Gram-neg + anaerobes | Alternative to Augmentin. Cefuroxime (2nd-gen cephalosporin) covers common enterics [8]. |
| Piperacillin-tazobactam | Very broad — Gram-pos, Gram-neg (including Pseudomonas), anaerobes | Reserved for severe/complicated intra-abdominal sepsis or hospital-acquired infections |
| Cephalosporin/Fluoroquinolone + Metronidazole | As above | Alternative regimens for inpatient diverticulitis [6] |
| Carbapenem (Meropenem/Imipenem) | Broadest — ESBL-producing Gram-neg, anaerobes | Reserved for critically ill patients, suspected resistant organisms, or tertiary peritonitis |
Timing of surgical prophylaxis [8]:
- At the induction of GA — to achieve high tissue concentration of antibiotics upon skin incision
- Additional dose if OT > 2× half-life (usually 3 hours for Augmentin/cefuroxime)
- Additional dose if blood loss > 1.5 L
Duration:
- Uncomplicated source (e.g., non-perforated appendicitis): Continue until 24h post-op [3]
- Complicated source (e.g., perforated appendicitis with abscess, generalised peritonitis): Continue 3–7 days post-op [3], guided by clinical response (resolution of fever, normalising WCC, improving clinically)
3. Tertiary Peritonitis
Tertiary peritonitis = persistent peritonitis after adequate initial therapy [3]. This is the most difficult to manage:
- Re-evaluate source control: Is there an ongoing source that was missed? Undrained abscess? Anastomotic leak? CT abdomen + pelvis to look for residual collections.
- Adjust antibiotics: Culture-guided — these patients often harbour resistant or opportunistic organisms (Staphylococcus, Enterococcus, Candida [2]):
- MRSA → Vancomycin or Linezolid
- VRE → Linezolid or Daptomycin
- Candida → Echinocandin (Caspofungin/Anidulafungin) or Fluconazole
- Pseudomonas → Piperacillin-tazobactam, Ceftazidime, or Carbapenem + Aminoglycoside
- Re-laparotomy / planned re-look laparotomy: May need repeat washouts every 24–48 hours
- Open abdomen management (laparostomy): In severe cases, the abdomen is left open with a temporary abdominal closure (vacuum-assisted closure / negative-pressure wound therapy) to allow ongoing drainage and repeated washouts
- ICU care: These patients are invariably in the ICU with multi-organ support
4. Specific Scenarios
Emergency Surgery for Diverticulitis-Related Peritonitis [6]
Indications for emergency surgery in diverticulitis [6]:
- Frank (free) perforation
- Failure of medical treatment with IV antibiotics
- Colonic obstruction
- Abscess failing non-operative intervention
Elective surgery (sigmoid colectomy with primary anastomosis — "interval colectomy") [6]:
- Previous complicated diverticulitis
- Immunocompromised patients
- Inability to exclude malignancy
Recurrent episodes of uncomplicated diverticulitis— Previously thought that risk of complications increases with recurrent disease but is now proven to be wrong [6]. Current guidelines no longer recommend elective surgery based on number of episodes alone.
Appendicitis with Peritonitis
Immediate surgery (present within 72h and fit for surgery) [3]:
- Laparoscopic appendicectomy is preferred — lower infection risk, less post-operative pain, shorter hospital stay
- Open surgery if gross sepsis or generalised peritonitis with haemodynamic instability
- Antibiotics: IV ceftriaxone + metronidazole (anaerobic coverage) [3]
- Non-complicated: continue until 24h post-op
- Complicated (abscess, phlegmon): continue 3–7 days post-op
Interval surgery (present > 72h and stable — walled-off appendix) [3]:
- IV antibiotics (~90% success rate) ± image-guided drainage of abscess
- Laparoscopic appendicectomy 6–8 weeks later
- Colonoscopy if > 40 years old to exclude caecal carcinoma [3]
Summary: Decision Framework
| Type | Is There a Surgical Source? | Primary Treatment | Key Antibiotics |
|---|---|---|---|
| Primary (SBP) | No | 3rd-gen cephalosporin (Cefotaxime/Ceftriaxone) × 5 days | Then secondary prophylaxis with Norfloxacin/Levofloxacin |
| Primary (CAPD) | No | IP 1st-gen cephalosporin + aminoglycoside (or 3rd-gen ceph) | Remove catheter if refractory/fungal/Pseudomonas |
| Primary (TB) | No | RIPE regimen × 6–9 months | No surgery unless complications |
| Secondary | Yes | Source control: surgery (laparotomy/laparoscopy) + peritoneal lavage | Broad-spectrum: Augmentin + Metronidazole or Pip-Taz |
| Tertiary | Persistent | Re-laparotomy, open abdomen, ICU | Culture-guided ± antifungals |
High Yield Summary — Management of Peritonitis
Immediate for ALL: IV fluids, NGT, urinary catheter, oxygen, pain relief, broad-spectrum antibiotics, close monitoring.
SBP: 3rd-gen cephalosporin (Cefotaxime/Ceftriaxone) × 5 days → stop when PMN < 250. IV albumin if renal impairment/high bilirubin. Lifelong fluoroquinolone prophylaxis (Norfloxacin/Levofloxacin).
CAPD peritonitis: IP 1st-gen cephalosporin + aminoglycoside (or 3rd-gen ceph). Start ASAP — don't wait for cultures. Continue PD unless refractory. Remove catheter if: refractory after 5 days / Pseudomonas / fungal. Success > 90%.
Secondary peritonitis: Source control is king — laparotomy/laparoscopy (PPU repair, appendicectomy, cholecystectomy, bowel resection). Percutaneous drainage for localised abscess. Broad-spectrum antibiotics (cover Gram-neg + Gram-pos + anaerobes). Hartmann's procedure for Hinchey III/IV diverticulitis.
Indications for urgent surgery: Peritonitis, pneumoperitoneum, strangulation, closed-loop obstruction, volvulus with peritoneal signs, incarcerated hernia.
Do NOT delay surgery in unstable patients with free gas + florid peritoneal signs. Do NOT wait for culture results before starting antibiotics. Do NOT use vancomycin as first-line for CAPD peritonitis.
Active Recall - Management of Peritonitis
1. What is the empirical antibiotic regimen for SBP, what is the duration, and what is the criterion for stopping treatment?
Show mark scheme
3rd-generation cephalosporin (Cefotaxime 2g IV Q8h or Ceftriaxone 2g IV daily). Duration: 5 days. Stop when ascitic fluid PMN < 250 cells/mm3. Also give IV albumin (1.5 g/kg day 1, 1 g/kg day 3) if renal impairment or bilirubin > 68 umol/L to prevent hepatorenal syndrome.
2. In CAPD peritonitis, what is the empirical antibiotic regimen, why is vancomycin avoided initially, and what are the 3 indications for Tenckhoff catheter removal?
Show mark scheme
Empirical: IP 1st-generation cephalosporin (cefazolin) + aminoglycoside (or 3rd-gen cephalosporin like ceftazidime to preserve residual renal function and avoid ototoxicity). Vancomycin avoided to minimise emergence of vancomycin-resistant strains (VRE). Catheter removal indications: (1) Refractory peritonitis with failure of effluent to clear after 5 days of antibiotics, (2) Peritonitis due to hydrophilic Gram-negative rods (e.g. Pseudomonas) - forms biofilms, (3) Fungal peritonitis (e.g. Candida) - antimicrobials insufficient with catheter in situ.
3. What are the 6 immediate resuscitative measures for a patient presenting with peritonitis, as listed in the lecture slides?
Show mark scheme
(1) IV fluid replacement (correct hypovolaemia from third-space losses), (2) Nasogastric tube (decompress stomach, prevent aspiration - paralytic ileus), (3) Urinary catheter (monitor urine output for fluid resuscitation), (4) Oxygen (prevent hypoxia from diaphragmatic splinting and sepsis), (5) Pain relief (IV opioids), (6) Broad-spectrum antibiotics (start empirically, do not wait for cultures). Plus close monitoring for change of condition.
4. Explain why Hartmann's procedure is preferred over primary anastomosis in Hinchey III/IV diverticular peritonitis.
Show mark scheme
In Hinchey III (generalised purulent peritonitis) and IV (faecal peritonitis), the peritoneal cavity is grossly contaminated. Performing a primary anastomosis (joining bowel ends together) in this environment carries a very high risk of anastomotic leak because tissues are inflamed, oedematous, and contaminated. Hartmann's procedure resects the affected segment, creates an end-colostomy (proximal stoma), and closes the distal rectal stump. The stoma can be reversed electively months later when the patient has recovered and inflammation has settled.
5. What is secondary prophylaxis for SBP, which agents are used, and what is the mechanism by which they prevent recurrence?
Show mark scheme
Lifelong fluoroquinolone prophylaxis (Norfloxacin 400mg PO daily or Levofloxacin 250mg PO daily) unless patient receives liver transplant with normalisation of liver function. Mechanism: selective intestinal decontamination - fluoroquinolones are incompletely absorbed by the gut, achieving high intraluminal concentrations. They have high activity against Gram-negative bacilli (the organisms that translocate to cause SBP) while preserving anaerobic flora, low bacterial resistance, and fewer side effects.
6. List 4 indications for emergency surgery in diverticulitis-related peritonitis and state one indication that is NO LONGER considered valid for elective sigmoid colectomy.
Show mark scheme
Emergency: (1) Frank free perforation, (2) Failure of medical treatment with IV antibiotics, (3) Colonic obstruction, (4) Abscess failing non-operative intervention. No longer valid: recurrent episodes of uncomplicated diverticulitis - previously thought risk of complications increases with recurrent disease but now proven wrong. Current guidelines do not recommend elective surgery based on number of episodes alone.
References
[1] Lecture slides: GC 195. Lower and diffuse abdominal pain RLQ problems; pelvic inflammatory disease; peritonitis and abdominal emergencies.pdf (p34, p39, p41, p42) [2] Senior notes: felixlai.md (Peritonitis treatment p741–743; SBP treatment and prophylaxis p449–450; CAPD peritonitis treatment p866–867; Case study p743) [3] Senior notes: maxim.md (Section 2.5 Peritonitis p46; Appendicitis management p181; Diverticulitis Hinchey classification and management p95, p194) [6] Senior notes: felixlai.md (Diverticulitis treatment p645–647; Emergency surgery indications p645; Hinchey staging p637) [7] Lecture slides: GC 194. Intestinal obstruction colorectal cancer.pdf (p25 — Indications for urgent surgery) [8] Senior notes: maxim.md (Surgical antibiotic prophylaxis p49)
Complications of Peritonitis
Peritonitis is dangerous not only because of the acute episode itself, but because it triggers a cascade of local and systemic complications that can kill the patient even after the source is controlled. Think of complications in two categories: systemic (the whole body is affected) and local (problems within the peritoneal cavity). I'll also cover complications specific to each subtype (SBP, CAPD, secondary) and post-operative complications.
A. Systemic Complications
These arise because the inflamed peritoneum (1.7 m² surface area) acts as a massive absorption surface for bacteria and endotoxins, while simultaneously sequestering litres of protein-rich fluid. The result is the simultaneous insults of hypovolaemia and sepsis.
1. Sepsis and Septic Shock
- Pathophysiology: Bacteria and endotoxins (lipopolysaccharide from Gram-negative cell walls) are absorbed across the inflamed peritoneum into the systemic circulation → activate the innate immune system → massive release of pro-inflammatory cytokines (TNF-α, IL-1, IL-6) → SIRS → if uncontrolled, progresses to sepsis → septic shock (persistent hypotension despite adequate fluid resuscitation, requiring vasopressors)
- Septicaemia, endotoxaemia leading to hypovolaemia & shock [1]
- Septic shock involves distributive shock (nitric oxide-mediated vasodilation → decreased SVR) superimposed on hypovolaemic shock (third-space losses)
- Mortality of septic shock from intra-abdominal source: 30–50%
2. Multi-Organ Failure (MOF)
When the sepsis cascade is not controlled, individual organ systems begin to fail sequentially. This is the terminal pathway of untreated peritonitis:
| Organ System | Complication | Mechanism |
|---|---|---|
| Lungs | ARDS (Acute Respiratory Distress Syndrome) | Inflammatory cytokines damage pulmonary capillary endothelium → increased permeability → non-cardiogenic pulmonary oedema → severe hypoxaemia. Also: abdominal distension pushes diaphragm up → basal atelectasis → further V/Q mismatch. |
| Kidneys | AKI (Acute Kidney Injury) | Pre-renal AKI from hypovolaemia (third-space losses) + sepsis-induced renal vasoconstriction + direct tubular injury from endotoxins. In SBP specifically, this manifests as hepatorenal syndrome (functional renal failure in cirrhosis triggered by SBP). [2] |
| Liver | Septic hepatitis / "shock liver" | Hypoperfusion + endotoxin-mediated intrahepatic cholestasis. In cirrhotic patients, SBP precipitates hepatic decompensation → worsening hepatic encephalopathy [2]. |
| Coagulation | DIC (Disseminated Intravascular Coagulation) | Endotoxins and cytokines activate the coagulation cascade diffusely → consumption of clotting factors and platelets → simultaneous microvascular thrombosis AND uncontrolled bleeding. Prolonged PT/APTT, low fibrinogen, raised D-dimers, low platelets. |
| Heart | Septic cardiomyopathy | Myocardial depression from circulating cytokines (TNF-α, IL-1) → reduced ejection fraction despite high cardiac output state. |
| Brain | Septic encephalopathy | Altered mental status — development of delirium, confusion and cognitive slowing — caused by infection and hepatic decompensation [2]. Cytokines cross the blood-brain barrier, alter neurotransmitter balance (increased GABA, decreased dopamine). |
MOF in Peritonitis — The Cascade
The typical sequence is: peritonitis → sepsis → lung failure (ARDS, often the first organ to fail) → kidney failure (AKI) → liver dysfunction → coagulopathy (DIC) → cardiovascular collapse → death. Each organ failure increases mortality by ~15–20%. By the time 3 organs have failed, mortality exceeds 70%.
3. Hypovolaemic Shock
- Sequestration of large amounts of protein-rich fluid into the peritoneal cavity [1]
- Paralytic ileus compounds this by causing further fluid sequestration within distended bowel loops
- If not aggressively resuscitated with IV fluids, the patient develops profound intravascular depletion → end-organ hypoperfusion → metabolic acidosis → cardiac arrest
- Associated electrolyte derangements: hypokalaemia (vomiting, NGT losses), metabolic acidosis (tissue hypoperfusion, lactate accumulation), hypoalbuminaemia (protein leak into peritoneal cavity)
4. Electrolyte and Metabolic Derangements
| Derangement | Mechanism |
|---|---|
| Metabolic acidosis | Tissue hypoperfusion → anaerobic metabolism → lactic acidosis. Also: AKI → impaired acid excretion. |
| Hypokalaemia | Vomiting, NGT losses, third-space sequestration. Dangerous: can cause cardiac arrhythmias and worsen paralytic ileus. |
| Hyponatraemia | Dilutional (ADH release in response to hypovolaemia) or from GI losses. |
| Hypoalbuminaemia | Massive protein leak across inflamed peritoneum. Worsens oedema (reduced oncotic pressure) and impairs drug binding. |
| Hyperglycaemia | Stress response → catecholamines and cortisol → insulin resistance. Infection will induce hyperglycaemia [2]. Particularly relevant in CAPD peritonitis with diabetic patients. |
| Hypoglycaemia | Nausea and anorexia of peritonitis will lead to malnutrition and hence hypoglycaemia in patients receiving hypoglycaemic agents or insulin [2]. A dual risk with hyperglycaemia in diabetics. |
B. Local (Intra-Abdominal) Complications
1. Intra-Abdominal Abscess
- The most common local complication of peritonitis
- Pathophysiology: The body's attempt to wall off infection is both protective and problematic. The omentum ("policeman of the abdomen") and fibrinous adhesions seal off pockets of infection, creating localised collections of pus. While this prevents generalised spread, the abscess itself becomes a persistent source of sepsis inaccessible to systemic antibiotics (poor penetration into the abscess cavity).
- Common sites: Subphrenic spaces (right and left), Morison's pouch, paracolic gutters, pelvis (pouch of Douglas), interloop collections
- Clinical features: Persistent fever (swinging/spiking pattern) despite appropriate antibiotics, persistent abdominal pain, leucocytosis, failure to improve. Should be suspected in patients with no improvement in abdominal pain or a persistent fever despite 3 days of antibiotic treatment [6]
- In appendicitis: Walled off → form appendiceal abscess → obstruction [3]. Not walled off → generalised peritonitis [3]
- Diagnosis: CT abdomen with contrast — rim-enhancing fluid collection ± air/debris
- Management: Percutaneous drainage of abdominal abscess [1] (CT-guided or USG-guided) + continued IV antibiotics. Surgical drainage if percutaneous approach fails or is not technically feasible.
- A special variant: pyogenic liver abscess — may develop due to spread of infection through the portal circulation [6] (e.g., from diverticulitis or appendicitis via pylephlebitis)
2. Paralytic Ileus
- Already present during peritonitis (reflex sympathetic inhibition + local mediators), but may persist after source control
- Prolonged ileus → continued third-space losses, inability to feed, risk of aspiration
- Differentiating prolonged ileus from early adhesive mechanical obstruction post-operatively can be challenging (CT with oral contrast helps)
3. Adhesion Formation
- Fibrinous exudates deposited during peritonitis organise into fibrous adhesions between bowel loops, omentum, and parietal peritoneum
- These adhesions are the most common cause of small bowel obstruction in the developed world
- Can cause obstruction weeks, months, or years after the original peritonitis episode
- Post-operative complications of surgery for peritonitis include: adhesive intestinal obstruction [2]
- Why does peritonitis cause MORE adhesions than a clean operation? Because the massive fibrin deposition overwhelms the normal fibrinolytic capacity of the peritoneal mesothelium
4. Fistula Formation
- Chronic inflammation/abscess adjacent to a hollow viscus can erode through the wall and create an abnormal communication (fistula)
- Particularly relevant in diverticulitis-related peritonitis [6]:
- Most commonly involve the bladder (colovesical fistula) — presents with pneumaturia, faecaluria, recurrent dysuria/UTI [3][6]
- Followed by vagina (colovaginal fistula) — especially in post-hysterectomy patients — presents with passage of faeces and flatus per vagina [6]
- Colocutaneous fistula — uncommon, usually easy to identify (faecal discharge from skin) [6]
- Coloenteric fistula — uncommon, may be asymptomatic or cause corrosive diarrhoea [6]
- Post-appendicectomy: Enterocutaneous fistula — result from an intraperitoneal abscess that fistulises to the skin [2]
- Management of fistula [6]: control sepsis by antibiotics and drainage → resection of affected segment of colon with primary anastomosis → simple repair of the secondarily involved organ (primary closure of bladder or vagina)
5. Intestinal Obstruction
Peritonitis can cause obstruction through multiple mechanisms:
| Mechanism | Type | Time Course |
|---|---|---|
| Paralytic ileus | Functional (no mechanical block) | Immediate — during and shortly after peritonitis |
| Adhesions | Mechanical SBO | Days to years after peritonitis |
| Inflammatory mass / phlegmon | Mechanical (external compression) | During acute episode |
| Stricture formation | Mechanical (luminal narrowing) | Chronic — recurrent attacks result in progressive fibrosis and scarring leading to intestinal strictures [6] |
- Partial colonic obstruction occurs because of relative luminal narrowing due to pericolonic inflammation or compression from a diverticular abscess [6]
- Complete colonic obstruction occurs because recurrent attacks of acute diverticulitis result in progressive fibrosis and scarring leading to formation of intestinal strictures [6]
- LBO due to fibrosis and stricture, SBO due to adhesion to inflamed bowel [3]
6. Wound Complications (Post-Operative)
Patients operated on for peritonitis have grossly contaminated surgical fields:
| Complication | Mechanism |
|---|---|
| Simple wound infection | Bacterial contamination of surgical wound from the infected peritoneal cavity. Incidence 20–40% in contaminated/dirty cases (vs. < 2% in clean surgery). Presents with wound erythema, discharge, dehiscence. [2] |
| Wound dehiscence (burst abdomen) | The combination of malnutrition, hypoalbuminaemia, infection, and raised intra-abdominal pressure (ileus, distension) impairs fascial healing → wound breakdown. Risk factors: emergency surgery, obesity, steroids, coughing. |
| Incisional hernia | Long-term consequence of impaired wound healing, especially after midline laparotomy for peritonitis. |
C. Complications Specific to Each Subtype
SBP-Specific Complications
| Complication | Mechanism |
|---|---|
| Hepatorenal syndrome (HRS) | SBP is one of the most common precipitants of HRS in cirrhotics. Infection → splanchnic vasodilation → effective hypovolaemia → renal vasoconstriction → functional renal failure. This is why IV albumin is given alongside antibiotics (to prevent HRS). Type 1 HRS has > 50% mortality. |
| Hepatic encephalopathy | Caused by infection and hepatic decompensation [2]. SBP increases ammonia production (bacterial urease activity) and impairs hepatic clearance → worsening encephalopathy. |
| Variceal haemorrhage | SBP can precipitate variceal bleeding by increasing portal pressure (splanchnic vasodilation → increased portal flow) and worsening coagulopathy. |
| Recurrence | ~70% recurrence rate at 1 year without secondary prophylaxis → this is why lifelong fluoroquinolone prophylaxis is essential [2]. |
| Death | In-hospital mortality of SBP: 20–30%. 1-year mortality after first episode: ~50–70% (reflects the severity of the underlying liver disease). SBP should prompt evaluation for liver transplantation. |
CAPD Peritonitis-Specific Complications
| Complication | Mechanism |
|---|---|
| Peritoneal membrane fibrosis / PD failure | Chronic exposure to dextrose-containing PD fluid and repeated peritonitis will cause fibrosis of peritoneal membrane leading to PD failure which is the major reason for conversion to long-term haemodialysis [2]. Each episode of peritonitis damages the mesothelial layer and promotes submesothelial fibrosis, eventually reducing the membrane's transport capacity. |
| Catheter-related complications | Exit-site infections, tunnel infections, catheter malposition, catheter blockage (omental wrapping) [2]. These often precede and precipitate peritonitis episodes. |
| Technique failure (conversion to HD) | Refractory or recurrent peritonitis is the single most common reason for transferring from PD to haemodialysis. |
| Encapsulating peritoneal sclerosis (EPS) | A rare but devastating complication of long-term PD ± repeated peritonitis. The peritoneum becomes encased in a thick fibrous "cocoon" that encases the bowel → intestinal obstruction. Presents with anorexia, nausea, abdominal pain, ascites, bowel obstruction. Diagnosis by CT (peritoneal calcification, bowel tethering). Management: tamoxifen + surgical enterolysis if severe. Mortality: 25–50%. |
Secondary Peritonitis-Specific Complications
These depend on the underlying cause but include all the local complications above plus:
| Complication | Detail |
|---|---|
| Anastomotic leak | Post-operative leak from bowel anastomosis → re-establishes peritoneal contamination → recurrent/persistent peritonitis. More common when primary anastomosis is performed in contaminated field. Primary anastomosis is NOT performed in cases of diffuse peritonitis, free perforation or if the patient is medically unstable [2] — precisely to avoid this complication. |
| Stoma-related complications | If Hartmann's procedure or defunctioning stoma is created: parastomal hernia, stoma prolapse, skin irritation, high-output stoma with dehydration, psychological impact. Stoma reversal itself carries 10–30% complication rate. |
| Pylephlebitis (septic portal vein thrombosis) | Associated with high fever, chills and rigors and jaundice. Thrombosis and infection within portal venous system. Caused by septicaemia in portal venous system and leads to development of intra-hepatic abscesses [2]. A rare but life-threatening complication, particularly of appendicitis and diverticulitis. |
| Progression to tertiary peritonitis | If source control is inadequate or the patient is immunosuppressed, secondary peritonitis may evolve into tertiary peritonitis with opportunistic organisms — the worst prognosis (mortality 30–60%). |
Diverticulitis-Specific Complications Leading to/From Peritonitis
The progression of diverticulitis complications is well-characterised [6][7]:
Complicated diverticulitis — perforation [7]:
- Resuscitation with IVF + antibiotics
- Percutaneous drainage for abscess
- Consider emergency surgery if:
- Unresponsive to antibiotics
- Septic shock (high fever, tachycardia, hypotension, oliguria)
- Generalised peritonitis (Hinchey III & IV)
The Hinchey classification stages represent progressive complications [7]:
| Stage | Description | Mortality |
|---|---|---|
| I | Confined pericolic or mesenteric abscess | 0% |
| II | Abscess confined to pelvis or retroperitoneum | 5% |
| III | Generalised purulent peritonitis | 25% |
| IV | Generalised faecal peritonitis | 50% |
Note how mortality escalates dramatically from stage II to IV — this underscores the importance of early diagnosis and intervention to prevent progression.
D. Summary of Complications by Time Course
| Timing | Complications |
|---|---|
| Immediate (hours) | Hypovolaemic shock, septic shock, metabolic acidosis, electrolyte derangements, paralytic ileus |
| Early (days) | ARDS, AKI, DIC, septic encephalopathy, intra-abdominal abscess, wound infection, hepatorenal syndrome (SBP), hepatic encephalopathy |
| Late (weeks–months) | Adhesive SBO, fistula formation, incisional hernia, PD membrane fibrosis, stoma complications, anastomotic stricture |
| Very late (months–years) | Recurrent SBP (without prophylaxis), encapsulating peritoneal sclerosis, adhesive SBO (can present years later) |
High Yield Summary — Complications of Peritonitis
Systemic: Sepsis → septic shock → MOF (ARDS → AKI → DIC → liver failure → cardiovascular collapse). Hypovolaemic shock from third-space losses. Electrolyte derangements (hypokalaemia, metabolic acidosis, hypoalbuminaemia).
Local (intra-abdominal): Intra-abdominal abscess (persistent fever despite antibiotics → CT → percutaneous drainage). Adhesion formation → SBO. Fistula formation (colovesical MC in diverticulitis). Paralytic ileus. Intestinal stricture.
SBP-specific: Hepatorenal syndrome (prevented by IV albumin), hepatic encephalopathy, variceal haemorrhage, 70% recurrence without prophylaxis, high 1-year mortality (prompts transplant evaluation).
CAPD-specific: Peritoneal membrane fibrosis → PD failure (MC reason for conversion to HD). Encapsulating peritoneal sclerosis (rare, devastating). Catheter complications.
Post-operative: Wound infection (20–40% in contaminated surgery), anastomotic leak (why primary anastomosis avoided in diffuse peritonitis), adhesive SBO, pylephlebitis, stoma complications.
Diverticulitis progression: Hinchey I (abscess, 0% mortality) → II (pelvic abscess, 5%) → III (purulent peritonitis, 25%) → IV (faecal peritonitis, 50%). Emergency surgery for Hinchey III–IV.
Active Recall - Complications of Peritonitis
1. Explain the pathophysiological cascade from peritonitis to multi-organ failure, naming at least 4 organ systems affected and the mechanism for each.
Show mark scheme
Peritonitis → bacterial/endotoxin absorption → SIRS → sepsis → MOF. (1) Lungs: ARDS from cytokine-mediated pulmonary capillary leak → non-cardiogenic pulmonary oedema. (2) Kidneys: AKI from hypovolaemia + sepsis-induced renal vasoconstriction + direct tubular injury. (3) Liver: septic hepatitis from hypoperfusion + endotoxin-mediated cholestasis. (4) Coagulation: DIC from diffuse activation of coagulation cascade → consumption of factors and platelets. (5) Brain: septic encephalopathy from cytokines crossing BBB. (6) Heart: septic cardiomyopathy from TNF-alpha and IL-1 causing myocardial depression.
2. What are the 3 SBP-specific complications, and why is IV albumin given alongside antibiotics in SBP?
Show mark scheme
SBP-specific complications: (1) Hepatorenal syndrome - SBP causes splanchnic vasodilation → effective hypovolaemia → renal vasoconstriction → functional renal failure. (2) Hepatic encephalopathy - infection increases ammonia production + impairs hepatic clearance. (3) Variceal haemorrhage - increased portal pressure and worsened coagulopathy. IV albumin (1.5 g/kg day 1, 1 g/kg day 3) is given to expand intravascular volume and prevent the renal vasoconstriction that causes HRS. Reduces mortality from ~30% to ~10%.
3. Why does repeated CAPD peritonitis lead to PD failure, and what is encapsulating peritoneal sclerosis?
Show mark scheme
Repeated peritonitis damages the mesothelial layer of the peritoneal membrane and promotes submesothelial fibrosis. Combined with chronic exposure to dextrose-containing PD fluid, this causes progressive peritoneal membrane fibrosis → reduced transport capacity → PD failure (most common reason for conversion to long-term haemodialysis). EPS is a rare but devastating complication where the peritoneum becomes encased in a thick fibrous cocoon encasing the bowel → intestinal obstruction. Presents with anorexia, nausea, abdominal pain, ascites, bowel obstruction. CT shows peritoneal calcification and bowel tethering. Treatment: tamoxifen + surgical enterolysis. Mortality 25-50%.
4. Why is primary anastomosis avoided in diffuse peritonitis, and what operation is performed instead for Hinchey IV diverticular peritonitis?
Show mark scheme
Primary anastomosis is avoided in diffuse peritonitis because the tissues are inflamed, oedematous, and contaminated → very high risk of anastomotic leak, which would re-establish peritoneal contamination. Hartmann's procedure is performed instead: resection of affected sigmoid with end-colostomy (proximal end brought out as stoma) and closure of distal rectal stump. The stoma is reversed electively months later. Hinchey IV (faecal peritonitis) carries 50% mortality.
5. A patient develops persistent spiking fever 5 days after laparotomy for perforated appendicitis despite appropriate antibiotics. What is the most likely complication and how would you investigate and manage it?
Show mark scheme
Most likely: intra-abdominal abscess. Suspect if no improvement in abdominal pain or persistent fever despite 3 days of antibiotics. Investigate with CT abdomen with contrast (rim-enhancing fluid collection with air or debris). Manage with CT-guided percutaneous drainage + continued IV antibiotics. If drainage fails or collection is not amenable to percutaneous approach, surgical drainage (re-laparotomy) is needed.
References
[1] Lecture slides: GC 195. Lower and diffuse abdominal pain RLQ problems; pelvic inflammatory disease; peritonitis and abdominal emergencies.pdf (p41–42) [2] Senior notes: felixlai.md (Peritonitis p738–743; SBP p449–450; CAPD peritonitis complications p865–867; Appendicitis complications p736–737; CRC anastomosis principles p695–696) [3] Senior notes: maxim.md (Appendicitis complications p179; Diverticulitis complications p194–195; Peritonitis p46) [6] Senior notes: felixlai.md (Diverticulitis complications p647; Fistula management p647; Obstruction mechanism p647) [7] Lecture slides: Diverticular diseases - Dr. J Tsang.pdf (p12–13 — Hinchey classification and complicated diverticulitis perforation management)